 | |
| Clinical data | |
|---|---|
| Trade names | 民得维 |
| Routes of administration | oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
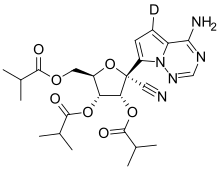
| Formula | C24H30DN5O7 |
| Molar mass | 502.546 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Deuremidevir, also known as VV116, is an nucleoside analogue antiviral drug. It is administrated through oral tablets, which contain the hydrobromide salt of this drug.[1]
Chemically speaking, the drug is a deuterated tri-isobutyrate of GS-441524, the active metabolite of remdesivir. It was first described in an 2020 preprint by a team including members of Wuhan Institute of Virology and Vigonvita.[2] It completed a phase 3 trial in 2022.[3] Junshi, which markets the drug, received conditional approval from China's National Medical Products Administration in January 2023.[4]
In November 2023, in response to viral mutations and changing characteristics of infection, the WHO adjusted its treatment guidelines. Among other changes, the use of deuremidevir was recommended against, except for clinical trials.[5]