Main types of initiation reaction
- Halogens undergo homolytic fission relatively easily. Chlorine, for example, gives two chlorine radicals (Cl•) by irradiation with ultraviolet light. This process is used for chlorination of alkanes.
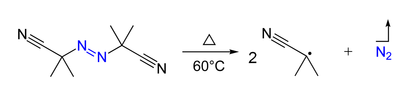
- Azo compounds (R-N=N-R') can be the precursor of two carbon-centered radicals (R• and R'•) and nitrogen gas upon heating and/or by irradiation. For example, AIBN and ABCN yield isobutyronitrile and cyclohexanecarbonitrile radicals, respectively.
- Organic peroxides each have a peroxide bond (-O-O-), which is readily cleaved to give two oxygen-centered radicals. The oxyl radicals are unstable and believed to be transformed into relatively stable carbon-centered radicals. For example, di-tert-butyl peroxide (t-BuOOt-Bu) gives two t-butoxy radicals (t-BuO•) and the radicals become methyl radicals (CH3•) with the loss of acetone. Benzoyl peroxide ((PhC)OO)2) generates benzoyloxyl radicals (PhCOO•), each of which loses carbon dioxide to be converted into a phenyl radical (Ph•). Methyl ethyl ketone peroxide is also common, and acetone peroxide is on rare occasions used as a radical initiator, too.
- Inorganic peroxides function analogously to organic peroxides. Many polymers are often produced from the alkenes upon initiation with peroxydisulfate salts. In solution, peroxydisulfate dissociates to give sulfate radicals:[3]
- [O3SO-OSO3]2− ⇌ 2 [SO4]−
The sulfate radical adds to an alkene forming radical sulfate esters, e.g. .CHPhCH2OSO3−, that add further alkenes via formation of C-C bonds. Many styrene and fluoroalkene polymers are produced in this way.
- In atom transfer radical polymerization (ATRP), carbon-halides reversibly generate organic radicals in the presence of transition metal catalyst.