This article relies excessively on references to primary sources. Please improve this article by adding secondary or tertiary sources. Find sources: "JZ-IV-10" – news · newspapers · books · scholar · JSTOR (June 2013) (Learn how and when to remove this template message)
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
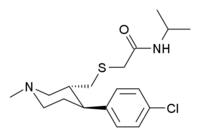
| Formula | C18H27ClN2OS |
| Molar mass | 354.94 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
JZ-IV-10 is a piperidine derivative related to cocaine which acts as a highly potent serotonin–norepinephrine–dopamine reuptake inhibitor (also called SNDRI, or triple reuptake inhibitor).[1] The eugeroic modafinil was used as a lead to fuel this compound's discovery.[2][3]