

An electron microscope is a microscope that uses a beam of electrons as a source of illumination. They use electron optics that are analogous to the glass lenses of an optical light microscope to control the electron beam, for instance focusing them to produce magnified images or electron diffraction patterns. As the wavelength of an electron can be up to 100,000 times smaller than that of visible light, electron microscopes have a much higher resolution of about 0.1 nm, which compares to about 200 nm for light microscopes.[1] Electron microscope may refer to:
Additional details can be found in the above links. This article contains some general information mainly about transmission electron microscopes.

Many developments laid the groundwork of the electron optics used in microscopes.[2] One significant step was the work of Hertz in 1883[3] who made a cathode-ray tube with electrostatic and magnetic deflection, demonstrating manipulation of the direction of an electron beam. Others were focusing of the electrons by an axial magnetic field by Emil Wiechert in 1899,[4] improved oxide-coated cathodes which produced more electrons by Arthur Wehnelt in 1905[5] and the development of the electromagnetic lens in 1926 by Hans Busch.[6] According to Dennis Gabor, the physicist Leó Szilárd tried in 1928 to convince him to build an electron microscope, for which Szilárd had filed a patent.[7]
To this day the issue of who invented the transmission electron microscope is controversial.[8][9][10][11] In 1928, at the Technical University of Berlin, Adolf Matthias (Professor of High Voltage Technology and Electrical Installations) appointed Max Knoll to lead a team of researchers to advance research on electron beams and cathode-ray oscilloscopes. The team consisted of several PhD students including Ernst Ruska. In 1931, Max Knoll and Ernst Ruska[12][13] successfully generated magnified images of mesh grids placed over an anode aperture. The device, a replicate of which is shown in the figure, used two magnetic lenses to achieve higher magnifications, the first electron microscope. (Max Knoll died in 1969, so did not receive a share of the 1986 Nobel prize for the invention of electron microscopes.)
Apparently independent of this effort was work at Siemens-Schuckert by Reinhold Rüdenberg. According to patent law (U.S. Patent No. 2058914[14] and 2070318,[15] both filed in 1932), he is the inventor of the electron microscope, but it is not clear when he had a working instrument. He stated in a very brief article in 1932[16] that Siemens had been working on this for some years before the patents were filed in 1932, claiming that his effort was parallel to the university development. He died in 1961, so similar to Max Knoll, was not eligible for a share of the 1986 Nobel prize.[17]
In the following year, 1933, Ruska and Knoll built the first electron microscope that exceeded the resolution of an optical (light) microscope.[18] Four years later, in 1937, Siemens financed the work of Ernst Ruska and Bodo von Borries, and employed Helmut Ruska, Ernst's brother, to develop applications for the microscope, especially with biological specimens.[18][19] Also in 1937, Manfred von Ardenne pioneered the scanning electron microscope.[20] Siemens produced the first commercial electron microscope in 1938.[21] The first North American electron microscopes were constructed in the 1930s, at the Washington State University by Anderson and Fitzsimmons [22] and at the University of Toronto by Eli Franklin Burton and students Cecil Hall, James Hillier, and Albert Prebus. Siemens produced a transmission electron microscope (TEM) in 1939.[23] Although current transmission electron microscopes are capable of two million times magnification, as scientific instruments they remain similar but with improved optics.
In the 1940s, high-resolution electron microscopes were developed, enabling greater magnification and resolution.[24] By 1965, Albert Crewe at the University of Chicago introduced the scanning transmission electron microscope using a field emission source,[25] enabling scanning microscopes at high resolution.[26] By the early 1980s improvements in mechanical stability as well as the use of higher accelerating voltages enabled imaging of materials at the atomic scale.[27][28] In the 1980s, the field emission gun became common for electron microscopes, improving the image quality due to the additional coherence and lower chromatic aberrations. The 2000s were marked by advancements in aberration-corrected electron microscopy, allowing for significant improvements in resolution and clarity of images.[29][30]
|
Main article: Transmission electron microscope |
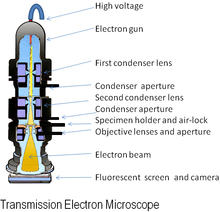
The original form of the electron microscope, the transmission electron microscope (TEM), uses a high voltage electron beam to illuminate the specimen and create an image. An electron beam is produced by an electron gun, with the electrons typically having energies in the range 20 to 400 keV, focused by electromagnetic lenses, and transmitted through the specimen. When it emerges from the specimen, the electron beam carries information about the structure of the specimen that is magnified by lenses of the microscope. The spatial variation in this information (the "image") may be viewed by projecting the magnified electron image onto a detector. For example, the image may be viewed directly by an operator using a fluorescent viewing screen coated with a phosphor or scintillator material such as zinc sulfide. A high-resolution phosphor may also be coupled by means of a lens optical system or a fibre optic light-guide to the sensor of a digital camera. Direct electron detectors have no scintillator and are directly exposed to the electron beam, which addresses some of the limitations of scintillator-coupled cameras.[31]
The resolution of TEMs is limited primarily by spherical aberration, but a new generation of hardware correctors can reduce spherical aberration to increase the resolution in high-resolution transmission electron microscopy (HRTEM) to below 0.5 angstrom (50 picometres),[32] enabling magnifications above 50 million times.[33] The ability of HRTEM to determine the positions of atoms within materials is useful for nano-technologies research and development.[34]
|
Main article: Scanning transmission electron microscopy |
The STEM rasters a focused incident probe across a specimen. The high resolution of the TEM is thus possible in STEM. The focusing action (and aberrations) occur before the electrons hit the specimen in the STEM, but afterward in the TEM. The STEMs use of SEM-like beam rastering simplifies annular dark-field imaging, and other analytical techniques, but also means that image data is acquired in serial rather than in parallel fashion.[35]
|
Main article: Scanning electron microscope |
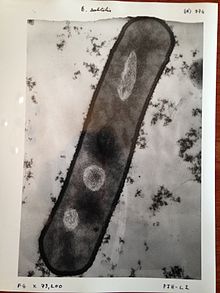
The SEM produces images by probing the specimen with a focused electron beam that is scanned across the specimen (raster scanning). When the electron beam interacts with the specimen, it loses energy by a variety of mechanisms. These interactions lead to, among other events, emission of low-energy secondary electrons and high-energy backscattered electrons, light emission (cathodoluminescence) or X-ray emission, all of which provide signals carrying information about the properties of the specimen surface, such as its topography and composition. The image displayed by SEM represents the varying intensity of any of these signals into the image in a position corresponding to the position of the beam on the specimen when the signal was generated.[36]
SEMs are different from TEMs in that they use electrons with much lower energy, generally below 20 keV,[37] while TEMs generally use electrons with energies in the range of 80-300 keV[38]. Thus, the electron sources and optics of the two microscopes have different designs, and they are normally separate instruments[39].
|
Main article: Electron diffraction |
Transmission electron microscopes can be used in electron diffraction mode where a map of the angles of the electrons leaving the sample is producdd. The advantages of electron diffraction over X-ray crystallography are primarily in the size of the crystals. In X-ray crystallography, crystals are commonly visible by the naked eye and are generally in the hundreds of micrometers in length. In comparison, crystals for electron diffraction must be less than a few hundred nanometers in thickness, and have no lower boundary of size. Additionally, electron diffraction is done on a TEM, which can also be used to obtain many other types of information, rather than requiring a separate instrument.[40][38]

|
See also: TEM Sample preparation, Ultramicrotomy, Staining, Cryofixation, Chemical milling, and Sputtering |
Materials to be viewed in a transmission electron microscope may require processing to produce a suitable sample. The technique required varies depending on the specimen and the analysis required:
In their most common configurations, electron microscopes produce images with a single brightness value per pixel, with the results usually rendered in greyscale.[56] However, often these images are then colourized through the use of feature-detection software, or simply by hand-editing using a graphics editor. This may be done to clarify structure or for aesthetic effect and generally does not add new information about the specimen.[57]
Electron microscopes are now frequently used in more complex workflows, with each workflow typically using multiple technologies to enable more complex and/or more quantitative analyses of a sample. A few examples are outlined below, but this should not be considered an exhaustive list. The choice of workflow will be highly dependent on the application and the requirements of the corresponding scientific questions, such as resolution, volume, nature of the target molecule, etc.
For example, images from light and electron microscopy of the same region of a sample can be overlaid to correlate the data from the two modalities. This is commonly used to provide higher resolution contextual EM information about a fluorescently labelled structure. This correlative light and electron microscopy (CLEM)[58] is one of a range of correlative workflows now available. Another example is high resolution mass spectrometry (ion microscopy), which has been used to provide correlative information about subcellular antibiotic localisation,[59] data that would be difficult to obtain by other means.
The initial role of electron microscopes in imaging two-dimensional slices (TEM) or a specimen surface (SEM with secondary electrons) has also increasingly expanded into the depth of samples.[60] An early example of these ‘volume EM’ workflows was simply to stack TEM images of serial sections cut through a sample. The next development was virtual reconstruction of a thick section (200-500 nm) volume by backprojection of a set of images taken at different tilt angles - TEM tomography.[61]
To acquire volume EM datasets of larger depths than TEM tomography (micrometers or millimeters in the z axis), a series of images taken through the sample depth can be used. For example, ribbons of serial sections can be imaged in a TEM as described above, and when thicker sections are used, serial TEM tomography can be used to increase the z-resolution. More recently, back scattered electron (BSE) images can be acquired of a larger series of sections collected on silicon wafers, known as SEM array tomography.[62][63] An alternative approach is to use BSE SEM to image the block surface instead of the section, after each section has been removed. By this method, an ultramicrotome installed in an SEM chamber can increase automation of the workflow; the specimen block is loaded in the chamber and the system programmed to continuously cut and image through the sample. This is known as serial block face SEM.[64] A related method uses focused ion beam milling instead of an ultramicrotome to remove sections. In these serial imaging methods, the output is essentially a sequence of images through a specimen block that can be digitally aligned in sequence and thus reconstructed into a volume EM dataset. The increased volume available in these methods has expanded the capability of electron microscopy to address new questions,[60] such as mapping neural connectivity in the brain,[65] and membrane contact sites between organelles.[66]

Electron microscopes are expensive to build and maintain. Microscopes designed to achieve high resolutions must be housed in stable buildings (sometimes underground) with special services such as magnetic field canceling systems.[67]
The samples largely have to be viewed in vacuum, as the molecules that make up air would scatter the electrons. An exception is liquid-phase electron microscopy[68] using either a closed liquid cell or an environmental chamber, for example, in the environmental scanning electron microscope, which allows hydrated samples to be viewed in a low-pressure (up to 20 Torr or 2.7 kPa) wet environment. Various techniques for in situ electron microscopy of gaseous samples have been developed.[69]
Scanning electron microscopes operating in conventional high-vacuum mode usually image conductive specimens; therefore non-conductive materials require conductive coating (gold/palladium alloy, carbon, osmium, etc.). The low-voltage mode of modern microscopes makes possible the observation of non-conductive specimens without coating. Non-conductive materials can be imaged also by a variable pressure (or environmental) scanning electron microscope.[citation needed]
Small, stable specimens such as carbon nanotubes, diatom frustules and small mineral crystals (asbestos fibres, for example) require no special treatment before being examined in the electron microscope. Samples of hydrated materials, including almost all biological specimens, have to be prepared in various ways to stabilize them, reduce their thickness (ultrathin sectioning) and increase their electron optical contrast (staining). These processes may result in artifacts, but these can usually be identified by comparing the results obtained by using radically different specimen preparation methods. Since the 1980s, analysis of cryofixed, vitrified specimens has also become increasingly used by scientists, further confirming the validity of this technique.[70][71][72]