| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| |
| |
| Properties | |
| XeOF4 | |
| Molar mass | 223.23 g/mol |
| Appearance | colorless liquid |
| Density | 3.17 g/cm3, liquid |
| Melting point | −46.2 °C (−51.2 °F; 227.0 K) |
| Reacts with water | |
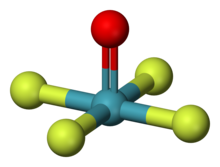
| Structure | |
| square pyramidal[1][2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Xenon oxytetrafluoride (XeOF
4) is an inorganic chemical compound. It is an unstable colorless liquid[2][3] with a melting point of −46.2 °C (−51.2 °F; 227.0 K)[4] that can be synthesized by partial hydrolysis of XeF
6, or the reaction of XeF
6 with silica[3] or NaNO
3:[5]
- NaNO
3 + XeF
6 → NaF + XeOF
4 + FNO
2
A high-yield synthesis proceeds by the reaction of XeF
6 with POF
3 at −196 °C (−320.8 °F; 77.1 K).[6]
Like most xenon oxides, it is extremely reactive, and it hydrolyses in water to give hazardous and corrosive products, including hydrogen fluoride:
- 2 XeOF
4 + 4 H
2O → 2 Xe + 8 HF + 3 O
2
In addition, some ozone and fluorine is formed.