| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Isoleucine
| |||
| Other names
2-Amino-3-methylpentanoic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.726 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H13NO2 | |||
| Molar mass | 131.175 g·mol−1 | ||
| Supplementary data page | |||
| Isoleucine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
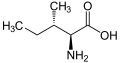
Isoleucine (abbreviated as Ile or I)[1] is an α-amino acid with the chemical formula HO2CCH(NH2)CH(CH3)CH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA.
With a hydrocarbon side chain, isoleucine is classified as a hydrophobic amino acid. Together with threonine, isoleucine is one of two common amino acids that have a chiral side chain. Four stereoisomers of isoleucine are possible, including two possible diastereomers of L-isoleucine. However, isoleucine present in nature exists in one enantiomeric form, (2S,3S)-2-amino-3-methylpentanoic acid.
Biosynthesis
As an essential amino acid, isoleucine is not synthesized in animals, hence it must be ingested, usually as a component of proteins. In plants and microorganisms, it is synthesized via several steps, starting from pyruvic acid and alpha-ketoglutarate. Enzymes involved in this biosynthesis include:[2]
- Acetolactate synthase (also known as acetohydroxy acid synthase)
- Acetohydroxy acid isomeroreductase
- Dihydroxyacid dehydratase
- Valine aminotransferase
Catabolism
Isoleucine is both a glucogenic and a ketogenic amino acid. After transamination with alpha-ketoglutarate the carbon skeleton can be converted into either Succinyl CoA, and fed into the TCA cycle for oxidation or conversion into oxaloacetate for gluconeogenesis (hence glucogenic). It can also be converted into Acetyl CoA and fed into the TCA cycle by condensing with oxaloacetate to form citrate. In mammals Acetyl CoA cannot be converted back to carbohydrate but can be used in the synthesis of ketone bodies or fatty acids, hence ketogenic.
Biotin, sometimes referred to as Vitamin B7 or Vitamin H, is an absolute requirement for the full catabolism of isoleucine (as well as leucine). Without adequate biotin, the human body will be unable to fully break down isoleucine and leucine molecules [3]. This can lead to numerous physiological issues (related to muscle maintenance and protein synthesis, lipid metabolism, and fatty acid metabolism) as well as cognitive issues resulting from general metabolic pathway failure and the irritating effects of hydroxyisovalerate, a byproduct of incomplete isoleucine catabolism. Isovaleric acidemia is an example of a disorder caused by incomplete catabolism of leucine.
Nutritional Sources
Even though this amino acid is not produced in animals, it is stored in high quantities. Foods that have high amounts of isoleucine include eggs, soy protein, seaweed, turkey, chicken, lamb, cheese, and fish.[4]
Isomers of isoleucine
| Forms of Isoleucine | |||||||
|---|---|---|---|---|---|---|---|
| Common name: | isoleucine | D-isoleucine | L-isoleucine | DL-isoleucine | allo-D-isoleucine | allo-L-isoleucine | allo-DL-isoleucine |
| Synonyms: | (R)-Isoleucine | L(+)-Isoleucine | (R*,R*)-isoleucine | alloisoleucine | |||
| PubChem: | CID 791 from PubChem | CID 94206 from PubChem | CID 6306 from PubChem | CID 76551 from PubChem | |||
| EINECS number: | |||||||
| CAS number: | 443-79-8 | 319-78-8 | 73-32-5 | 1509-35-9 | 1509-34-8 | 3107-04-8 | |
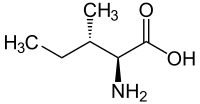  |
| L-isoleucine (2S,3S) and D-isoleucine (2R,3R) |
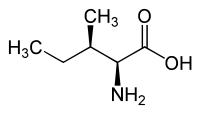  |
| L-allo-isoleucine (2S,3R) and D-allo-isoleucine (2R,3S) |
Synthesis
Isoleucine can be synthesized in a multistep procedure starting from 2-bromobutane and diethylmalonate.[5] Synthetic isoleucine was originally reported in 1905.[6]
German chemist Felix Ehrlich discovered isoleucine in hemoglobin in 1903.
References
- ^ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Retrieved 2007-05-17.
- ^ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ^ http://www.metametrix.com/learning-center/case-studies/2004/biotin-detoxification-needs-in-cognitively-delayed-adult
- ^ [1], List is in order of highest to lowest of per 200 Calorie serving of the food, not volume or weight.
- ^ "dl-Isoleucine". Organic Syntheses. 1955; Collected Volumes, vol. 3, p. 495.
- ^ Bouveault and Locquin, Compt. rend., 141, 115 (1905).
External links
| General topics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| By properties |
| ||||||||||