| |

| |
| Names | |
|---|---|
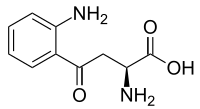
| Preferred IUPAC name
(2S)-2-Amino-4-(2-aminophenyl)-4-oxo-butanoic acid | |
| Other names
(S)-Kynurenine
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| MeSH | Kynurenine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H12N2O3 | |
| Molar mass | 208.217 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
l-Kynurenine is a metabolite of the amino acid l-tryptophan used in the production of niacin.
Kynurenine is synthesized by the enzyme tryptophan dioxygenase, which is made primarily but not exclusively in the liver, and indoleamine 2,3-dioxygenase, which is made in many tissues in response to immune activation.[1] Kynurenine and its further breakdown products carry out diverse biological functions, including dilating blood vessels during inflammation[2] and regulating the immune response.[3] Some cancers increase kynurenine production, which increases tumor growth.[1]
Kynurenine protects the eye by absorbing UV light, especially in the UVA region (315-400 nm).[4] Kynurenine is present in the lens and retina as one of multiple tryptophan derivatives produced in the eye, including 3-hydroxykynurenine, that together provide UV protection and aid in enhancing visual acuity.[5][6] The use of kynurenine as a UV filter is consistent with its photostability and low photosensitization, owing to its efficient relaxation from the UV-induced excited state.[7] The concentration of this UV filter decreases with age,[8] and this loss of free kynurenine and the concomitant formation of relatively more photosensitizing kynurenine derivatives and kynurenine-protein conjugates may contribute to the formation of cataracts.[9][10][11]
Evidence suggests that increased kynurenine production may precipitate depressive symptoms associated with interferon treatment for hepatitis C.[12] Cognitive deficits in schizophrenia are associated with imbalances in the enzymes that break down kynurenine.[13] Blood levels of kynurenine are reduced in people with bipolar disorder.[14] Kynurenine production is increased in Alzheimer's disease[15] and cardiovascular disease[16] where its metabolites are associated with cognitive deficits[17] and depressive symptoms.[18] Kynurenine is also associated with tics.[19][20]
Kynureninase catabolizes the conversion of kynurenine into anthranilic acid[21] while kynurenine-oxoglutarate transaminase catabolizes its conversion into kynurenic acid. Kynurenine 3-hydroxylase converts kynurenine to 3-hydroxykynurenine.[22]
Kynurenine has also been identified as one of two compounds that makes up the pigment that gives the goldenrod crab spider its yellow color.[23]
