- The following is an archived discussion of a featured article nomination. Please do not modify it. Subsequent comments should be made on the article's talk page or in Wikipedia talk:Featured article candidates. No further edits should be made to this page.
The article was promoted by Graham Colm (talk) 09:17, 15 August 2014 [1].
Fluorine (edit | talk | history | links | watch | logs)
| Toolbox |
|---|
- Dean of the Chemistry Department: Parcly Taxel (+R8R Gtrs) 04:39, 7 July 2014 (UTC)[reply]
Fluorine. Atomic number 9. The lightest halogen, most reactive element, an extreme challenge to isolate… yet it is found everywhere from toothpaste to uranium enrichment plants. I've been working from the suggestions of FAC 3 and Sandbh, fixing the article refs and performing a whole-article copyedit. I've also introduced a few new references to fill in unreferenced statement gaps. So here we go. Fourth time lucky, eh? ![]() Parcly Taxel 04:39, 7 July 2014 (UTC)[reply]
Parcly Taxel 04:39, 7 July 2014 (UTC)[reply]
Harvard errors (resolved)
[edit]The HarvErrors script suggests that 21 of the references listed in the Indexed references section are not cited. These should be deleted or moved to a Further reading section.Aa77zz (talk) 10:48, 7 July 2014 (UTC)[reply]
- Done in double quick time. Parcly Taxel 11:32, 7 July 2014 (UTC)[reply]
- Still one to sort out: Audi, G.; Bersillon, O.; Blachot, J.; Wapstra, A. H. (2003). --Mirokado (talk) 23:48, 11 July 2014 (UTC)[reply]
- That is not an error. I used the ((NUBASE 2003)) template in the citation, which HarvErrors mistakes for a redundant citation when in fact it is cited in the Isotopes section. Parcly Taxel 00:27, 12 July 2014 (UTC)[reply]
- I have fixed the template and the article. --Mirokado (talk) 12:16, 13 July 2014 (UTC)[reply]
- That is not an error. I used the ((NUBASE 2003)) template in the citation, which HarvErrors mistakes for a redundant citation when in fact it is cited in the Isotopes section. Parcly Taxel 00:27, 12 July 2014 (UTC)[reply]
- Still one to sort out: Audi, G.; Bersillon, O.; Blachot, J.; Wapstra, A. H. (2003). --Mirokado (talk) 23:48, 11 July 2014 (UTC)[reply]
John (support)
[edit]I have never seen the word monoisotopy before. Is it a real word? --John (talk) 21:57, 7 July 2014 (UTC)[reply]
- Sure it is. @R8R Gtrs: mentioned it in the article's talk page as part of his pre-FAC check, all but one of whose suggestions I've weaved into the article. Parcly Taxel 23:24, 7 July 2014 (UTC)[reply]
- Are you sure? It doesn't seem to exist on the Internet except on a couple of Wikipedia articles. --John (talk) 08:45, 8 July 2014 (UTC)[reply]
- Even I think "monoisotopy" is a highly technical term. It's been swapped out now. Parcly Taxel 10:16, 8 July 2014 (UTC)[reply]
- I just copied the words from the part of the article I quoted. I am clearly not to be the judge of how Englsih words are formed/used, since I wasn't born in any English-speaking country, and I haven't lived in any, nor am I now.--R8R (talk) 21:01, 8 July 2014 (UTC)[reply]
- Even I think "monoisotopy" is a highly technical term. It's been swapped out now. Parcly Taxel 10:16, 8 July 2014 (UTC)[reply]
- Are you sure? It doesn't seem to exist on the Internet except on a couple of Wikipedia articles. --John (talk) 08:45, 8 July 2014 (UTC)[reply]
I've taken it out. Here's another nitpick:
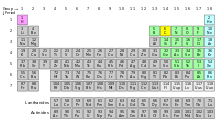
What is this table actually for? It does not mention halogens, though they are discussed in the article and its subject is one. Are noble gases also non-metals? Etc. --John (talk) 09:31, 8 July 2014 (UTC)[reply]
- This is a byproduct of the cumulative edits between the last FAC 3 years prior and now. From what you've said I also think that noble gases should come before nonmetals in the compounds section. That has been done, along with the legend's removal: the latter is all too redundant. Parcly Taxel 10:16, 8 July 2014 (UTC)[reply]
- This table is for a clearer difering the sets of elements discussed under each subheader. For example, antimony is mentioned among nonmetals, even though it is chemically closer to metals. The reader is explained what the borders between groups of elements under each header are via that table. Earler, those unobvious parts were explained in a note; that note is gone now for some reason. I'll get it back, compare with what we have now. Also, regarding the categories of the picture: each category of elements on the picture (metals, nonmetals, noble gases, hydrogen, carbon) coresponds to a category described in a subsection (Metals, Other nonmetals, Noble gases, Hydrogen, Organic chenistry). I hope that makes the purpose of the picture clear. Since it was obviously not as clear as intended (because of the missing note), I'll get the note back; if it's still unclear, please help make it more understandable.
- Regarding titles: they are secondary to facts described under them. And it makes most sense (as I see it) to go in the order of hydrogen -- metals -- nonmetals -- noble gases -- organic chemistry. Then we can adjust the titles to the story. For example, nonmetals (other than noble gases and hydrogen) can be called "other reactive nonmetals," and the meaning is still clear. Or, if that's not good enough, we can add a line written in italics under the header explaining that, similar to ((redirect)). There are options.--R8R (talk) 21:01, 8 July 2014 (UTC)[reply]
- Yep, the legend is back as a note; I've touched up the English as well. And then I realise we need more relentless critics, so this nomination won't stall. BANZAI! Parcly Taxel 04:01, 9 July 2014 (UTC)[reply]
- Regarding titles: they are secondary to facts described under them. And it makes most sense (as I see it) to go in the order of hydrogen -- metals -- nonmetals -- noble gases -- organic chemistry. Then we can adjust the titles to the story. For example, nonmetals (other than noble gases and hydrogen) can be called "other reactive nonmetals," and the meaning is still clear. Or, if that's not good enough, we can add a line written in italics under the header explaining that, similar to ((redirect)). There are options.--R8R (talk) 21:01, 8 July 2014 (UTC)[reply]
- I think I now support. Nice work; here are the trifling copyedits I performed. --John (talk) 23:04, 22 July 2014 (UTC)[reply]
Sandbh (support)
[edit]I'm reasonably satisfied that this article meets all the FAC criteria bar (1a) prose; (2c) consistent citations; and (3) media. On prose I made some edits to the lead however User:Parcly Taxel has rewritten some of these, more or less back to the way they were. That is fine, however I remain personally dissatisfied with the standard of prose. Given any more of my edits to prose may be rewritten I will stop here. May I ask another editor to review the prose, in case I am being too pernickety? Re 2c, consistent citations, I have not looked closely at these to determine if they are satisfactory (I did see some Lide 2004 citations which need a pp rather than a p; and Burney 1999, which needs a p rather than a pp). Media looks OK but I haven't checked copyright status. 05:56, 13 July 2014 (UTC)[reply]
- Eh, tillie. I "reverted" some of those copyedits (@Sandbh: yours) because they actually made the prose look worse, not better (indeed, I noticed the fragmented nature of the sentences introduced – stoppy-movey). John knows better; I've left his copyedits as they were. As for the single and double p-letters, yes, that has been fixed. Parcly Taxel 09:29, 13 July 2014 (UTC)[reply]
- Noted and understood. I look forward to further developments. Sandbh (talk) 12:10, 13 July 2014 (UTC)[reply]
- As for the media, none of them are fair use, the authors are properly cited and everything's fine under the sun and moon. FACR#3 is all smiles. Parcly Taxel 09:34, 13 July 2014 (UTC)[reply]
Lede
- "…the rest is converted into corrosive hydrogen fluoride, a precursor to various organic fluorides and the critical aluminium refining flux cryolite.'
- The location of cryolite as the first word in a five word descriptive phrase is too far away. By the time you get to the end of the phrase it doesn't make sense.
- Changed to read "...organic fluorides and cryolite, the critical aluminium refining flux": is this better? Double sharp (talk) 11:35, 16 July 2014 (UTC)[reply]
- Need a semicolon after fluorides Sandbh (talk) 11:53, 16 July 2014 (UTC)[reply]
- Changed to read "...organic fluorides and cryolite, the critical aluminium refining flux": is this better? Double sharp (talk) 11:35, 16 July 2014 (UTC)[reply]
- The location of cryolite as the first word in a five word descriptive phrase is too far away. By the time you get to the end of the phrase it doesn't make sense.
- "Organofluorine compounds persist in the environment due to the strength of the carbon–fluorine bond, but the potential health impact of such compounds is unclear."
- The use of but in this sentence doesn't make sense; there is nothing needing to be said that needs a 'but'.
- "A few plants and bacteria synthesize organofluorine poisons to deter herbivores, but fluorine has no known metabolic role in mammals."
- Use of a 'but' here is awkward. Sandbh (talk) 11:24, 16 July 2014 (UTC)[reply]
- Changed these two consecutive sentence to read "Organofluorine compounds persist in the environment due to the strength of the carbon–fluorine bond. The potential health impact of such compounds is unclear: a few plants and bacteria synthesize organofluorine poisons to deter herbivores, though fluorine has no known metabolic role in mammals." Is this better? Double sharp (talk) 11:35, 16 July 2014 (UTC)[reply]
- Somewhat although the second sentence mixes concepts. Joining the concept of 'health impact' to the concept of plant-generated herbivore poisons is a long bow; and then to add that fluorine has no metabolic roles in mammals is another obscure connection. Sandbh (talk) 11:53, 16 July 2014 (UTC)[reply]
- Changed these two consecutive sentence to read "Organofluorine compounds persist in the environment due to the strength of the carbon–fluorine bond. The potential health impact of such compounds is unclear: a few plants and bacteria synthesize organofluorine poisons to deter herbivores, though fluorine has no known metabolic role in mammals." Is this better? Double sharp (talk) 11:35, 16 July 2014 (UTC)[reply]
- Use of a 'but' here is awkward. Sandbh (talk) 11:24, 16 July 2014 (UTC)[reply]
Electron configuration
- "…two electrons in a filled inner shell and seven in an outer shell one short of completion."
- Last phrase, 'outer shell one short of completion' is grammatically awkward.
- "Fluorine's first ionization energy is third-highest among all elements, behind helium and neon, so removing electrons from neutral fluorine atoms is very difficult.
- The 'so' does not read well.
- "Fluorine has a high electron affinity, second only to chlorine, preferring to capture an electron and become isoelectronic with the noble gas neon…
- Sentence doesn't read well: finishing with 'affinity' and starting on the other side with 'preferring' doesn't flow very well. Sandbh (talk) 11:34, 16 July 2014 (UTC)[reply]
- All problems above this line have been fixed. Parcly Taxel 09:23, 17 July 2014 (UTC)[reply]
Reactivity
- "The bond energy of difluorine is much lower than those of Cl2 and Br2, similar to that of a weak peroxide bond, which accounts for its high reactivity and easy dissociation."
- Awkward due to mixing of quantity tenses (singular value of F; mutiple values of Cl and Br). Suggest: "The bond energy of difluorine is much lower than that of either Cl2 or Br2, and is similar to that of a weak peroxide bond; this accounts for its high reactivity and easy dissociation."
- "Bonds to other atoms are very strong because of its high electronegativity."
- The 'its' does not make sense; the EN of fluorine is that of the F atom, not difluorine.
- "Reactions of elemental fluorine with metals require varying conditions: alkali metals cause explosions and alkaline earth metals display vigorous activity in bulk, but most other metals such as aluminium and iron must be powdered to prevent metal fluoride layers from passivating, and noble metals require pure fluorine gas at 300–450 °C (575–850 °F). Metalloids and some solid nonmetals (sulfur, phosphorus, and selenium) burn with a flame in room temperature fluorine. Hydrogen sulfide and sulfur dioxide combine readily with fluorine, the latter sometimes explosively, but sulfuric acid exhibits much less activity."
- The "buts" are awkward. Both can be replaced by semicolons. The order of metal fluoride layers and passivating is the wrong way round: "to prevent passivation due to the formation of metal fluoride layers" is the sense of what needs to be said.
- "Carbon dioxide and carbon monoxide react at room and slightly higher temperatures.."; "...and other organic chemicals beget..."
- Suggest: "Carbon dioxide reacts at room temperature; carbon monoxide at a slightly higher temperature"
- Kill the 'beget'; replace with a more modern word
- "nitrogen requires electric discharge and elevated temperatures for reaction"
- Suggest: "nitrogen requires an electric discharge"
- "ammonia's reaction is potentially explosive"
- Awkward construction. Suggest: "ammonia may react explosively."
- "Oxygen does not combine under ambient conditions, but can be made to using electric discharge at low temperatures and pressures…"
- Oxygen does not combine with what? But can be made "to" what? Sandbh (talk) 01:22, 19 July 2014 (UTC)[reply]
- You know what? Just put all your prose mistakes here and say that you'll support once everything is resolved. I want this article through and promoted as fast as possible. The problems in Reactivity have all been fixed. Parcly Taxel 06:07, 19 July 2014 (UTC)[reply]
@FAC coordinators: @Sandbh: Done now with your copyedits? I'd love to have this article promoted quickly to avoid stalling – we've got so much support for all the criteria now. Parcly Taxel 23:46, 25 July 2014 (UTC)[reply]
- Oh. No. Not done. I have
eightthreezero sections to go. Given the other supports, there is no need necessarily to wait for me, subject to the views of the FAC coordinators. I support in spirit, in any event, as per my earlier comments. I'm confident I'll be able to address all of my prose concerns. Sandbh (talk) 00:24, 26 July 2014 (UTC)[reply]- I've finished my section copyedits; and checked the lede, the notes and the captions. Sandbh (talk) 03:51, 29 July 2014 (UTC)[reply]
- поддержка (Support!) Sandbh (talk) 05:03, 29 July 2014 (UTC)[reply]
- I've finished my section copyedits; and checked the lede, the notes and the captions. Sandbh (talk) 03:51, 29 July 2014 (UTC)[reply]
Hurricanehink (support)
[edit]Support. I stumbled here from my own FAC, and was quite pleased by the article! Just a few comments.
- US$15 - this should have a link to USD. Throughout the article, you're inconsistent whether you use $ or US$
- Organofluorine should perhaps be linked in the last paragraph of the lead
- "Fluorine has high reactivity because compared with Cl2 and Br2, difluorine's bond energy is much lower, similar to a weak peroxide bond,[18][19] allowing elemental fluorine to dissociate easily. " - I get what it means, but I think it could be explained better with a better sentence structure
- I can only suggest separating the "allowing ..." part into a separate sentence, and a slight rewording, which I did, but it would be nice if someone else gave it a look.
- I reworded the sentence, now it's two sentences and reads all fine. Parcly Taxel 00:24, 15 July 2014 (UTC)[reply]
- I can only suggest separating the "allowing ..." part into a separate sentence, and a slight rewording, which I did, but it would be nice if someone else gave it a look.
- "Fluorine is highly toxic." - this sentence is pretty short. Perhaps add "to living organisms" afterward, to give it a bit more girth?
- The short sentence makes the statement stronger, doesn't it? Regardless, doesn't the word "toxic" already imply that the toxicity is a property only living organisms interact with? (I can't word it better, sorry.) I mean, wouldn't "toxic to living organisms" be just the same as "toxic" (and thus also a tautology)?
- No, and some people use it when referring to (poisoning) a catalyst. Nergaal (talk) 12:07, 15 July 2014 (UTC)[reply]
- I get the idea, statement has been "inflated" for clarity per the original request. Now! @Nergaal, Hurricanehink, John, Aa77zz, and Sandbh:! You support the nomination? Parcly Taxel 23:06, 15 July 2014 (UTC)[reply]
- No, and some people use it when referring to (poisoning) a catalyst. Nergaal (talk) 12:07, 15 July 2014 (UTC)[reply]
- Make sure you link ppm and explain what it is.
- "−188 °C" - this and all other temperatures should have conversion to Fahrenheit per WP:ACCESS
- Note 5 looks like the image should be first, with the text following, but right now it looks messy
- I did what I could, set the image before the text. It's the best thing that came to my mind.
- "Hydrofluoric acid, aqueous hydrogen fluoride," - I don't think a comma is appropriate here. I think you should either add "acid, which is aqueous...", or make it a dash.
- "3 kg" - in lbs?
- When was the "Montreal Protocol"?
All in all, a good read! ♫ Hurricanehink (talk) 18:06, 14 July 2014 (UTC)[reply]
- Thank you! Your comments are valid and good (really), so I followed them except where noted.--R8R (talk) 20:03, 14 July 2014 (UTC)[reply]
- Excellent, thanks for the quick replies, they all look great! ♫ Hurricanehink (talk) 02:02, 16 July 2014 (UTC)[reply]
Mirokado (support)
[edit]Just a few comments.
Reactivity:- "a weak peroxide bond": as opposed to a strong peroxide bond, or intending to clarify that peroxide bonds are weak? I think the latter, in which case "the weak peroxide bond" would be clearer
"nitrogen requires an electric discharge at elevated temperatures for reaction due to its very strong triple bonds" The N2 molecule has one triple bond, so I think it will be better to use the singular: "nitrogen requires an electric discharge at elevated temperatures for reaction due to its very strong triple bond"
- Occurrence / Earth: There is no explanation of the thousand-fold increase in relative abundance between the universe and Earth : how does this relate to the mentioned cosmic rarity of fluorine compared to neighbouring elements?
- Explanation not required. Rare in the universe does not necessarily mean rare in Earth's crust. @R8R Gtrs:: mind if you wanna pull out a ref of some sort for this?
- This comes from two reasons: fluorine's reactivity, which makes fluorine, which leaves stars and comes to Earth, chemically react with rocks, after which fluorine becomes a part of rocks and doesn't leave the Earth; and the fact fluorine which stays in stars is not safe there, and may undergo nuclear reactions with other elements (which makes it not fluorine anymore). Besides, the rarity of fluorine in stars is explained, with this very argument :) --R8R (talk) 11:07, 20 July 2014 (UTC)[reply]
- Compounds / Metals: "Alkaline earth difluorides possess strong ionic bonds as well but are insoluble": I find myself asking, why?
- No need to ask about that. There already is a reference and that's freely available – have a look at it. That's what they were for, after all.
- This once was in the article when it was 90 KB of text alone and was cut when making the article have a readable size; the reason is low (high absolute values, but negative) lattice energies, which are lower (larger absolute values, but negative) than sums of hydration energies, making it more favorable to stay undissolved, as the undissolved matter has the lowest internal energy. (I remember, I was intrigued with this at some point as well). I understand why you're asking, but I wouldn't add this, as this seems more of a detail, and this is an overview article.--R8R (talk) 11:07, 20 July 2014 (UTC)[reply]
Environmental concerns / Biopersistence: "... since their biological metabolisms are hard." : sounds clumsy, how about: "since they are not readily biologically degraded"Biological role / Natural biochemistry: I couldn't find a nice simple definition for "ω-fluoro" by following the fatty acids wl or googling, can you clarify somehow?Note 17: Perhaps it would be better to use the French spelling for Nicklès since it changes the pronounciation a lot: either Jérôme Nicklès or his full name François Joseph Jérôme Nicklès (I looked here while checking this)
-- Mirokado (talk) 00:58, 20 July 2014 (UTC)[reply]
- @Mirokado: Fixed all problems you mentioned, with accompanying commentary. Parcly Taxel 01:39, 20 July 2014 (UTC)[reply]
- Thanks for the quick response. I've left the two points where where I think the content could be a bit more stand-alone in case we can think of any change, but they are pretty minor and should not affect featured article status, so supporting now. -- Mirokado (talk) 02:41, 20 July 2014 (UTC)[reply]
Peter Isotalo (toxicity, headings; support)
[edit]An informative, well-illustrated and ambitious article. Some things are not quite clear to me, though:
The first thing I tried to look up was what makes fluorine toxic in what I assume is its pure form, and at the same time beneficial to oral hygiene. This information is somewhat disjointed and slightly contradictory, though. Under "Toxicity" it's claimed to be toxic. Period. No qualifications, but much later "Biological role" explains exactly how it isn't toxic. Later still, "Precautions" goes back to toxicity again. I understand that there's a difference between various compounds and whatnot, but the article isn't quite stating the obvious.
- Would you try to explain one more time, I'm not sure I get it? I specified under "Toxicity" that elemental fluorine is toxic, because the whole "Characteristics" section discusses elemental fluorine only, as done with any other element article; "Biological role" discusses (obviously) some organic compounds, and "Precautions" discusses effects of HF and fluoride ions. There is no contradiction I see; but would you point me to it?
- "Elemental" helps, but why not point out in the section heading as well? "Elemental characteristics" perhaps? If the standard formula is unclear, it doesn't hurt tweaking it.
- If "Biological role" is actually about organic compounds, why isn't it under "Organic compounds"? Does that also mean that "Industry and applications" is about inorganic chemistry? This may be obvious to you, but not to everyone.
- Peter Isotalo 14:07, 20 July 2014 (UTC)[reply]
- As explained below, this disjoint information problem should have been solved with my restructuring of sections. In particular the parts saying that fluorine is toxic and those indicating otherwise have been split into distant sections. Parcly Taxel 14:39, 21 July 2014 (UTC)[reply]
Also, I don't understand the table of contents. Why are medical and agricultural applications placed under "Biological role" rather than "Industry and applications"? They're very clearly about industrial applications, not biology per se. The sub-heading "Natural biochemistry" hints at the rather artificial separation of the biochemical industry from steel, polymers and whatnot. And why the manual-like "Precautions"? Why isn't this grouped with "Toxicity" or the likes? Peter Isotalo 11:32, 20 July 2014 (UTC)[reply]
- I understand what you mean. Sandbh's framework of the article which I copyedited really was disjointed in several places. Now I'm considering a substantial rearrangement of sections based on the zinc article:
- Two sections of applications, the medicinal and the industrial (the latter includes agriculture), since we have so much.
- Unification of the Toxicity subsection from Characteristics, the Biological role section and the precautions – there's so little about them.
- …and in fact, I've done it already. I think it makes the contents more coherent; what do you think Isotalo? Parcly Taxel 10:03, 21 July 2014 (UTC)[reply]
- I hesitate to put precautions, HF (aq), and F− (aq) under "Biological role": they're more about toxicity than about F playing a positive role in biochemistry. Also I would not call the section "natural occurrence", which sounds to me as though it was talking about F in the earth's crust, the Solar System, or something (which is already above).
- Since F is the subject of the article, the primary focus, I feel, should be the element (F2). So I think the scheme should be that toxicity without qualification is on F2, and if you are talking about other F compounds, you must have the qualification.
- I agree with moving the medicinal and agricultural applications out of "biological role".
- I've made a couple of edits to make this new scheme better. Nevertheless, I would like to also hear what R8R has to say on this. Double sharp (talk) 03:03, 22 July 2014 (UTC)[reply]
- I am too late to see any changes being done, but I think the page as of now is pretty fine (except I moved the Environmental concerns section from between Medical applications and Biological role to the bottom, as the former clearly wasn't the best place to keep it, between two interrelated sections) --R8R (talk) 21:56, 23 July 2014 (UTC)[reply]
- Would you give me a couple of days so I could get a great reference book I have so I could check it and (thereafter) think what should be done best?--R8R (talk) 12:55, 20 July 2014 (UTC)[reply]
- No rush on my account.
- Peter Isotalo 13:30, 20 July 2014 (UTC)[reply]
- Minor issue, really, but rearranging the information improves the article quite a bit. Support.
- Peter Isotalo 05:58, 23 July 2014 (UTC)[reply]
FAC coord notes (reference and image reviews)
[edit]Coincidentally, Parcly pinged the FAC coords for an assessment just as I was looking over the review and adding the following:
- Looks like image licensing and source reviews needed -- will list at WT:FAC.
- It also looks like this would be the first (potentially successful) FAC nom for both Parcly and R8R, so I'd like to see a spotcheck of sources for accuracy and avoidance of close paraphrasing; will likewise request at WT:FAC.
- There are many duplicate links in the article. Given its length and technical nature, some may well be justified but pls review. With Polytetrafluoroethylene, for instance, we not only get three links but also duplicate abbreviations "(PTFE)" in the first two instances and then a brand name "(Teflon)" for the last, although the brand name had already been mentioned. You can use this script to highlight the repeated links.
- It was done once before, but it's good that you noted it, since, yes, that has shown to be useful, thanks. I've removed all duplicate links, except for a few compound links in the Compounds (where they totally make sense) and Industrial applications (when discussing some (not all mentioned) compounds with a role in the text) sections. The word "Teflon" is mentioned twice, once just standing alone in parentheses when mentioning the history of it, and once mentioned in Industrial applications. Both times, the word is relevant to the text surrounding it (industry). Is this not okay? (As for the other two points, it's for an external reviewer to judge, not us, but this has been looked at previously, so I think it should be mostly or even completely fine)--R8R (talk) 00:59, 26 July 2014 (UTC)[reply]
Cheers, Ian Rose (talk) 23:53, 25 July 2014 (UTC)[reply]
Source review, all points resolved at 06:56, 29 July 2014 (UTC) - spotchecks not done
- Quote-initial and -terminal ellipses should be omitted
- Done
- FN22, 27, 174: pages?issues
- Done; for sources where I couldn't easily find those placed, I've replaced them (also changing the numbering scheme)
- Gribble: are there two editors or just one?
- Done
- As I understand it, PRWeb doesn't author their content, they just publish it
- Currently, not the reports as such serve as refs, but the PRWeb pages that link to the reports. I think this is okay?
- Be consistent in when you include publisher location
Removed all of them – they are made redundant by the publisher anyway.Location details are now included unless the source is available online. Sandbh (talk) 03:48, 29 July 2014 (UTC)[reply]
- Newspaper titles like Boston Globe should be italicized
- I have checked ref formatting. I have not found any further mistakes in format except for the Chiste ref, which was corrected
- European Commission: ordering of the directorate name affects alphabetization - with the European part first, this is out of order
- I've moved the European part to the end, because the directorate part is more important
- Brantley formatting is quite strange
- Done
- Burney: be consistent in how initials are punctuated
- Done
- Be consistent in whether/when you include states for US locations, and whether these are abbreviated
- No mention of the state or country anywhere. Thank you.
- Be consistent in whether you include publisher for periodicals
- Done. Sandbh (talk) 03:48, 29 July 2014 (UTC)[reply]
- Check alphabetization of references list
- I've checked, a few swaps were required, thank you
- Meyer: location formatting
- Done
- Preskorn: can you verify that this title is correct?
- I've corrected the title, thank you
- The two dissertations are formatted quite differently
- Are the Lagow and the Rhoades refs meant? If so, they look quite similar and both use ((cite book))
- Now formatted consistently. Sandbh (talk) 04:08, 29 July 2014 (UTC)[reply]
- Are the Lagow and the Rhoades refs meant? If so, they look quite similar and both use ((cite book))
- Sidgwick: use title caps
- Done
- Why do some refs have month and year in the first set of parentheses, while others have only year and then a later set with month?
- Done
Nikkimaria (talk) 04:09, 26 July 2014 (UTC)[reply]
- @Sandbh and R8R Gtrs:
You're to blame for this.I need you now! (And the issues with 0 after them have been fixed.) Parcly Taxel 07:13, 26 July 2014 (UTC)[reply]- I'll add this to my list and cheerfully decline your serving of blame-pie :) Sandbh (talk) 11:55, 26 July 2014 (UTC)[reply]
- I've replaced bold zeros with "Done" messages _only_because_ it's easier to see what's been done and what's not that way. The remaining part should be easier. (Also, you got me, why are Sandbh and I to blame? Reply on my talkpage, please)--R8R (talk) 01:26, 27 July 2014 (UTC)[reply]
- Now the unresolved issues are bolded; that's a better idea. And I'm going to call out for all the FAC reviewers here. @Hamiltonstone, Secret, Dirac66, Dan56, Dank, Sandbh, and Farrtj: if you're pinged here, you're needed here. I need a thorough image review and reference spot-check. And maybe @R8R Gtrs: can join in the fun as well – I'm the nominator, not him. Parcly Taxel 07:45, 27 July 2014 (UTC)[reply]
- Let's say I will not :) Also, I de-bolded two "kind of complete" issues -- I think they are done, but would want some confirmation. One issue left -- doing great so far--R8R (talk) 07:07, 28 July 2014 (UTC)[reply]
- Actually we've got two issues to do, not one. My edit on making locations consistent got reverted by S&bh. Parcly Taxel 22:58, 28 July 2014 (UTC)[reply]
- I understood there was only one (?) reference missing a location, of the references normally provided with locations, which I added as part of the reversion. Sandbh (talk) 23:10, 28 July 2014 (UTC)[reply]
- Resolved as per above comments. Sandbh (talk) 03:48, 29 July 2014 (UTC)[reply]
- I understood there was only one (?) reference missing a location, of the references normally provided with locations, which I added as part of the reversion. Sandbh (talk) 23:10, 28 July 2014 (UTC)[reply]
- Actually we've got two issues to do, not one. My edit on making locations consistent got reverted by S&bh. Parcly Taxel 22:58, 28 July 2014 (UTC)[reply]
- Let's say I will not :) Also, I de-bolded two "kind of complete" issues -- I think they are done, but would want some confirmation. One issue left -- doing great so far--R8R (talk) 07:07, 28 July 2014 (UTC)[reply]
- Now the unresolved issues are bolded; that's a better idea. And I'm going to call out for all the FAC reviewers here. @Hamiltonstone, Secret, Dirac66, Dan56, Dank, Sandbh, and Farrtj: if you're pinged here, you're needed here. I need a thorough image review and reference spot-check. And maybe @R8R Gtrs: can join in the fun as well – I'm the nominator, not him. Parcly Taxel 07:45, 27 July 2014 (UTC)[reply]
Partial image review. Too many for me to do the whole lot.
- File:Liquid fluorine tighter crop.jpg - derivative of an OTRS ticketed work - looks OK
- File:Fluorine shielding.svg - derivative of Commons-licenced work - looks OK
- File:Beta fluorine unit cell.svg - looks OK
- File:Fluorite-270246.jpg - OTRS ticketed looks OK
- File:Apatite Canada.jpg - looks OK
- File:Ivigtut cryolite edit.jpg - looks OK
- File:Book9-25.gif - reproduction of out-of-coyright work - looks OK
- File:Recherches sur l’isolement du fluor, Fig. 5.PNG - as above
- File:Henri Moissan HiRes.jpg - as above
- File:Uranium hexafluoride crystals sealed in an ampoule.jpg - US PD licence - looks OK
- File:Sodium-fluoride-unit-cell-3D.png - looks OK
- File:Bismuth-pentafluoride-chain-from-xtal-1971-3D-balls.png - looks OK
- File:Rhenium-heptafluoride-3D-balls.png - looks OK
- File:Chlorine-trifluoride-3D-balls.png - looks OK
- File:Xenon tetrafluoride crop.gif - US PD licence - looks OK
- File:FluorocarbonCrabFish.JPG - looks OK
Can someone else go on from here? Ta. hamiltonstone (talk) 09:48, 27 July 2014 (UTC)[reply]
Continued image review by Curly Turkey
[edit]- File:Fluorine cell room.jpg released to Public Domain under an ORTS
- File:SF6 current transformer TGFM-110 Russia.jpg CC-by-SA by uploader/photographer
- File:A water droplet DWR-coated surface2 edit1.jpg CC-by-SA by uploader/photographer
- File:US Navy 090526-F-1333S-023 A service member embarked aboard the Military Sealift Command hospital ship USNS Comfort (T-AH 20) gives a Fluoride treatment to a patient during a Continuing Promise 2009 medical civil service projec.jpg US government work, thus Public Domain
- File:Prozac pills.jpg CC-by-SA by uploader/photographer
- File:PET-MIPS-anim.gif released to Public Domain by uploader/creator
- File:Gifblaar.jpg CC-by-SA with OTRS
- File:HF burned hands.jpg CC-by-SA with OTRS
- File:Future ozone layer concentrations.gif NASA image, thus PD
- File:PFOS-3D-vdW.png assuming the uploader really created this, it's okay, but could be tagged in a more helpful manner (Information tag)
- File:Periodic table fluorine.svg released to PD by original creator and modifier
- File:Nafion2.svg We should assume that if File Upload Bot (Magnus Manske) uploaded this from de.wp with a CC-by-SA licence, that's because that was the licence on the original upload?
- Chemical formulas are no subject for copyright (thus in public domain). I dared therefore to change the license.
- Okay, I didn't realize chemical formulas were inelegible for copyright. This one's fine now. Curly Turkey ⚞¡gobble!⚟ 21:04, 1 August 2014 (UTC)[reply]
- Chemical formulas are no subject for copyright (thus in public domain). I dared therefore to change the license.
- File:Goretex photo.png source pointed to is (a) Wikipedia (b) a deleted page. What was the original source? Who owns the copyright?
- Unfortunately, I can't tell. I've removed the picture.
- File:1080PoisonWarning gobeirne.png derived from this sign. How could this not be under copyright? At any right, the original copyright owner (a government?) has not been indicated.
- I have found a very similar image, here, which clearly features the logo of New Zealand's Department of Conservation. Is it (or a reproduction) considered appropriate? ALternatively, the website of the said Department feataures blank templates for these warning signs, for example [2]. I believe such templates are fine with our copyright policy (given they're found on the Department's official website), aren't they?
- That depends on the country. Under US law, government creations are automatically PD, but the US s the exception there. It's not true in Canada or Japan, for instance. Unless you can find something that explicitly states these things should be PD, it should be assumed they're under copyright. Crown copyright affirms this (scroll down to New Zealand). Curly Turkey ⚞¡gobble!⚟ 21:16, 1 August 2014 (UTC)[reply]
- You seem to be right about New Zealand's copyright. I haven't found anything on why that shouldn't be copyrighted, so I've removed the picture.
- That depends on the country. Under US law, government creations are automatically PD, but the US s the exception there. It's not true in Canada or Japan, for instance. Unless you can find something that explicitly states these things should be PD, it should be assumed they're under copyright. Crown copyright affirms this (scroll down to New Zealand). Curly Turkey ⚞¡gobble!⚟ 21:16, 1 August 2014 (UTC)[reply]
- I have found a very similar image, here, which clearly features the logo of New Zealand's Department of Conservation. Is it (or a reproduction) considered appropriate? ALternatively, the website of the said Department feataures blank templates for these warning signs, for example [2]. I believe such templates are fine with our copyright policy (given they're found on the Department's official website), aren't they?
- File:DOT hazmat signs - Fluorine.svg can the "creator" validly claim copyright to release to Public Domain? These should be Public Domain in the first place, as they're faithful recreations of US Department of Transportation images
- I agree this should be in PD. I've contacted the uploader, but if he doesn't reply, isn't it okay to change the license to PD by ourselves, for these images are in PD, each one of them? (If not, there's a way out, ((multiple images)), which would help create
((multiple image | footer = U.S. hazard signs for commercially transported fluorine<ref name="NOAA data sheet">[[#NOAASheet|National Oceanic and Atmospheric Administration]].</ref> | width = 90 | image1 = DOT hazmat class 6.1.svg | alt1 = A diagonal placard with warning poison | image2 = DOT hazmat class 5.1.svg | alt2 = A diagonal placard with warning corrosive | image3 = DOT hazmat class 8.svg | alt3 = A diagonal placard with warning inhalant | image4 = DOT hazmat class 2.3 (alt).svg | alt4 = A diagonal placard with warning oxidant ))
- ) I don't know which is better.
- You might want to go with
((multiple image))until it's sorted out. There may be some claim for copyright in the arrangement of images or something. Curly Turkey ⚞¡gobble!⚟ 21:04, 1 August 2014 (UTC)[reply]- Okay, I've changed that picture to ((multiple image)). I don't really expect an answer, given the last edit from that account was done in 2013.--R8R (talk) 22:33, 1 August 2014 (UTC)[reply]
- You might want to go with
- ) I don't know which is better.
- File:Schmelzflusselektrolyse von Aluminium.svg it says it was derived from an original by Andreas Schmidt. What evidence is there that Schmidt has made this free?
- I suspect this is not subject for PD either per this. Am I right? If so, I'll change that as well.
- Under PD-textlogo? It's not a logo, and not very simple. Curly Turkey ⚞¡gobble!⚟ 21:04, 1 August 2014 (UTC)[reply]
- But the text of the template doesn't require it to be a logo? (however, I am unsure what the criteria for simplicity are. I've asked the German uploader about Schmidt, and am waiting for him to reply)
- An image on the HH process article is explicitly freely licenced. Maybe that will work @Curly Turkey:? (It's PNG, but I have the skills to vectorise it. Tell me if SVG would be preferred for this article. @Ceranthor: get over here and do some reference spotchecking. Parcly Taxel 09:30, 2 August 2014 (UTC)[reply]
- Whether it's SVG or PNG makes no difference to FAC---I think it's a matter of optimizing file size. The file's not pretty, but if it bugs you, you can hunt around for someone to make something prettier---but it's sufficient for an FA. Curly Turkey ⚞¡gobble!⚟ 09:58, 2 August 2014 (UTC)[reply]
- I made the vector version myself and it is now live. Parcly Taxel 04:46, 3 August 2014 (UTC)[reply]
- Whether it's SVG or PNG makes no difference to FAC---I think it's a matter of optimizing file size. The file's not pretty, but if it bugs you, you can hunt around for someone to make something prettier---but it's sufficient for an FA. Curly Turkey ⚞¡gobble!⚟ 09:58, 2 August 2014 (UTC)[reply]
- An image on the HH process article is explicitly freely licenced. Maybe that will work @Curly Turkey:? (It's PNG, but I have the skills to vectorise it. Tell me if SVG would be preferred for this article. @Ceranthor: get over here and do some reference spotchecking. Parcly Taxel 09:30, 2 August 2014 (UTC)[reply]
- But the text of the template doesn't require it to be a logo? (however, I am unsure what the criteria for simplicity are. I've asked the German uploader about Schmidt, and am waiting for him to reply)
- Under PD-textlogo? It's not a logo, and not very simple. Curly Turkey ⚞¡gobble!⚟ 21:04, 1 August 2014 (UTC)[reply]
- I suspect this is not subject for PD either per this. Am I right? If so, I'll change that as well.
———Curly Turkey ⚞¡gobble!⚟ 11:06, 1 August 2014 (UTC)[reply]
Source spotchecking
[edit]- Comment - I notice I was pinged about a source review. I'm away, but if no one comments in the next day or two, I'll see what I can do. Sorry I can't be of more help. ceranthor 12:00, 2 August 2014 (UTC)[reply]
- @Ceranthor: Nikki has obliged with a source review for formatting/relaiability but if you could spotcheck a few sources for accurate use and avoidance of close paraphrasing, that'd be great. Cheers, Ian Rose (talk) 04:56, 8 August 2014 (UTC)[reply]
I checked twelve sources from the 11:32, 6 August 2014 version, starting with #11 and going up in increments of 11. I found one one apparently unsubstantiated source (#55); one minor doi inconsistency (#66a); and one missing page number (#77a). The first of these anomalies may represent a misplaced source, given the extensive development and copy editing this article has been subject to. The second and third anomalies are not serious. Overall, this appears to be a satisfactory spot check, although I will take advice from the FAC coordinator on this point. Conflict of interest declaration: I participated in the copy-editing of this article; I am a member of WikiProject Elements. Sandbh (talk) 13:22, 13 August 2014 (UTC)[reply]
Source: #11, Cheng et al. 1999
Claim: the magnetic ordering (susceptibility) of fluorine is 1.2×10−4
Source check: Confirmed
Source: #22a, Wiberg, Wiberg & Holleman 2001, p. 404
Claim: "Some solid nonmetals (sulfur, phosphorus) react vigorously in liquid air temperature fluorine."
Source check: "Sulfur and phosphorus react vigourously with fluorine at liquid air temperature."
Conclusion: Confirmed; it is a close paraphrased sentence in my opinion, but it is attributed.
Source: #33, Emeléus & Sharpe 1974, p. 111
Claim: "Oxygen does not combine with fluorine under ambient conditions, but can be made to using electric discharge at low temperatures and pressures; the products tend to disintegrate into their constituent elements when heated."
Source check: "The synthesis of various oxygen fluorides has been accomplished by flow reactions via electric discharge methods at low tempertures and low reactant pressures."
Conclusion: Confirmed to the extent of no inconsistency, noting there are two other sources for this claim. No close paraphrasing.
Source: #44, Müller 2009
Claim: For further detail on the concept of disorder in crystals, see the referenced general reviews
Check: Confirmed
Source: #55, Lodders 2003
Claim: "Fluorine is the thirteenth most common element in Earth's crust at 600–700 ppm (parts per million) by mass."
Check: Anomalous: source makes no reference to crystal abundances, as far as I can see?
Source: #66a, Schmedt, Mangstl & Kraus 2012
Claim: "The existence of gaseous fluorine in crystals, suggested by the smell of crushed antozonite, is contentious."
Check: Confirmed; no close paraphrasing detected. The reference is to the English version of the article whereas the doi given (doi:10.1002/ange.201203515) is for German edition; for consistency the doi should be doi:10.1002/anie.201203515
Source: #77a, Kirsch 2004
Claim: "Andreas Sigismund Marggraf first characterized it [Hydrofluoric acid] in 1764 when he heated fluorite with sulfuric acid, and the resulting solution corroded its glass container"
Check: Confirmed; no close paraphrasing evident. The page numer (2) is missing.
Source: #88a, Okazoe 2009
Claim: "The Frigidaire division of General Motors experimented with chlorofluorocarbon refrigerants in the late 1920s, and Kinetic Chemicals was formed as a joint venture between GM and DuPont in 1930 hoping to market Freon-12 (CCl2F2) as one such refrigerant. It replaced earlier and more toxic compounds, increased demand for kitchen refrigerators, and became profitable; by 1949 DuPont had bought out Kinetic and marketed several other Freon compounds"
Check: Confirmed, to the extent of no inconsistency, noting there are three other sources for this claim; no close paraphrasing detected.
Source: #99a, Pauling 1960, pp. 454–464
Claim: "Hydrogen and fluorine combine to yield hydrogen fluoride, in which discrete molecules form clusters via hydrogen bonding. Hydrogen fluoride thus behaves more like water than hydrogen chloride."
Check: Possibly OK; I cannot see all of the pages of the source; no inconsistency or paraphrasing detected.
Source: #110, Babel & Tressaud 1985, pp. 91–96
Claim: "Rare earth elements and many other metals form mostly ionic trifluorides."
Check: I can see five of the six pages listed. Confirmed to the extent of no inconsistency, noting there are two other sources for this claim; no close paraphrasing detected.
Source: #121, Lide 2004, pp. 4.72, 4.91, 4.93
Claim: "Covalent bonding first comes to prominence in the tetrafluorides: those of zirconium, hafnium and several actinides are ionic with high melting points, while those of titanium, vanadium, and niobium are polymeric, melting or decomposing at no more than 350 °C (660 °F)."
Check: Confirmed, no close paraphrasing detected
Source: #132, Chang & Goldsby 2013, p. 706
Claim: Boron trifluoride is planar and possesses an incomplete octet. It functions as a Lewis acid and combines with Lewis bases like ammonia to form adducts
Check: Cannot access source; comparison with an earlier edition of Chang confirms content; no close paraphrasing detected.
- Supplementary spot-check
As I had already ordered two tomes at the British Library, and as the nominator has asked me to add any further comments I might have about spot checks, let me add that I have checked refs 18, 58, 85, 102, 118, 120, 122 in Greenwood and Earnshaw and all are OK; in the same book I checked ref 25 but found no mention of hydrogen on that page (though as a layman I may simply have missed it); ref 102 is OK, though with a page range of five pages it took a bit of finding; I could not check ref 125, because it is too vague; and I flatly decline to check ref 140 – it is too much to ask anyone to wade through a total of twelve pages. I seem to have ordered the wrong version of Wiberg et al – what I have before me was published in 1995 – and so I am afraid I can't check any of the refs to the 2001 book. – Tim riley talk 11:46, 16 August 2014 (UTC)[reply]
Comments by Dank
[edit]As always, feel free to revert my copyediting. - Dank (push to talk)
- I copyedited the article per my standard disclaimer. These are my edits. - Dank (push to talk) 21:59, 4 August 2014 (UTC)[reply]
- Thank you very much for your edits! They are useful and nice, as far as I can judge.--R8R (talk) 08:53, 5 August 2014 (UTC)[reply]
- I groan when I hit chemistry articles, they're way over my head, but this one was very lively and readable, thanks for that.
- That was the intention; nice to know it worked.--R8R (talk) 08:53, 5 August 2014 (UTC)[reply]
- "themselves scheduled for substitution by 2030–2040 by hydrofluorocarbons (HFCs) with no chlorine and zero ODP. In 2007 this date was brought forward to 2020": If substitution is mandated (where?) by 2020, then it's not scheduled for 2030–2040. (2030? 2040? different dates in different countries?) Also, if everyone has agreed to 2020 already, then I'm not sure if the later dates are interesting enough to include.
- Yes, a good catch. 2020 is when the developed countries have to complete the phaseout; developing countries have to complete the phaseout by 2030, with a small share allowed to be used until 2040.--R8R (talk) 08:53, 5 August 2014 (UTC)[reply]
- "it should first be rinsed under a jet of water for 10–15 minutes to prevent further damage and any clothing worn should be removed": A more encyclopedic tone would be something like: further damage can be reduced by rinsing it under a jet of water for 10–15 minutes and removing contaminated clothing.
- Agree. I've changed that sentence to: "If skin has been exposed to HF, damage can be reduced by rinsing it under a jet of water for 10–15 minutes and removing contaminated clothing."--R8R (talk) 08:53, 5 August 2014 (UTC)[reply]
- Just a note that I'm personally agnostic on "[with] noun plus -ing" following a comma, but many style guides, a lot of the reviewers, and one of the FAC coords don't like it, so I've reworded throughout. (For instance, "production peaking" became "peaking in production".)
- Soliciting opinions: I changed the % in "supplying 6% of the global population" to "percent". WP:% isn't clear on whether to switch over to "percent" in more narrative text, after using "%" in more scientific contexts.
- "metspar": This word appears to be used in only two Wikipedia articles, so consider substituting "metallurgical grade fluorspar" (both times it occurs in the text). If you'd rather not, then don't italicize it, and I'd recommend either defining it as metallurgical grade fluorspar at first occurrence, or creating a stub and linking the term. In addition, using a phase like "metspar grade" would at least give the reader a clue to the meaning (although there's an argument that that's redundant). Same goes for "acidspar".
- The other article is a spin-off of this one (just in case you're interested). I've removed italicization; just before the words "acidspar" and "metspar" were first introduced, they were described as "grades," which I changed to "metallurgical grades." I believe that is okay?--R8R (talk) 08:53, 5 August 2014 (UTC)[reply]
- Sure, good enough. - Dank (push to talk)
- The other article is a spin-off of this one (just in case you're interested). I've removed italicization; just before the words "acidspar" and "metspar" were first introduced, they were described as "grades," which I changed to "metallurgical grades." I believe that is okay?--R8R (talk) 08:53, 5 August 2014 (UTC)[reply]
- The text says "An enzyme that binds fluorine to carbon – Adenosyl-fluoride synthase – was discovered in bacteria in 2002." This isn't sufficient to support the statement in the lead section: "a few plants and bacteria synthesize organofluorine poisons to deter herbivores."
- But the sentence: "The most common is fluoroacetate, which is used as a defense against herbivores by at least 40 plants in Africa, Australia and Brazil." is?--R8R (talk) 08:53, 5 August 2014 (UTC)[reply]
- No, because it doesn't indicate that the bacteria use it as a defense against herbivores. - Dank (push to talk) 13:21, 5 August 2014 (UTC)[reply]
- Removed the statement of bacteria in the lead – they are only mentioned in one sentence in the corresponding section. Parcly Taxel 07:09, 6 August 2014 (UTC)[reply]
- No, because it doesn't indicate that the bacteria use it as a defense against herbivores. - Dank (push to talk) 13:21, 5 August 2014 (UTC)[reply]
- But the sentence: "The most common is fluoroacetate, which is used as a defense against herbivores by at least 40 plants in Africa, Australia and Brazil." is?--R8R (talk) 08:53, 5 August 2014 (UTC)[reply]
- Most wikiprojects object to multiple uses of "US$", and many don't even use "US$" the first time. I'm not familiar with your wikiproject's thoughts on this; fill me in? My read of the first bullet point at WP:MOS#Currencies is that this isn't okay per MOS, and therefore not okay at FAC.
- My impression is opposite. I believe the first use is regulated, and the following ones are not at all. A reader from any other country using dollars might find multiple repetition of "US$" useful, as they won't even accidentally confuse it with their own dollars even though it was once shown U.S. dollars were used. Also, what if a reader does not wish to read the whole article, looking for some specific info only? The wikiproject has no standard on this.--R8R (talk) 08:53, 5 August 2014 (UTC)[reply]
- Well, we've also got WP:$ to contend with: "In general, the first mention of a particular currency should use its full, unambiguous signifier (e.g. A$52), with subsequent references using just the appropriate symbol (e.g. $88), unless this would be unclear." I'll be happy to ask around at a few wikiprojects if you like, but I've rarely seen this at GA, A or FA level. - Dank (push to talk)
- My impression is opposite. I believe the first use is regulated, and the following ones are not at all. A reader from any other country using dollars might find multiple repetition of "US$" useful, as they won't even accidentally confuse it with their own dollars even though it was once shown U.S. dollars were used. Also, what if a reader does not wish to read the whole article, looking for some specific info only? The wikiproject has no standard on this.--R8R (talk) 08:53, 5 August 2014 (UTC)[reply]
These are some of the changes I made while copyediting. I'd appreciate knowing if anyone can think of any time we wouldn't want to change the "before" phrase into something like the "after" phrase:
- en route: not italicized, since it has entered the language (SOED, m-w.com)
- Given lighter elements show greater abundances, fluorine's value ... is exceptional: other elements ... are twenty or more times as common.: Among the lighter elements, fluorine's abundance value ...
- its role as an additive to: its role as an additive in
- [A sentence with two semicolons separating three independent clauses]: [lose one of the semicolons]
- About 180,000 metric tons of fluoropolymers and over US$3.5 billion revenue per year were made in 2006–2007: ..., generating over US$3.5 billion revenue per year.
- rooves: roofs
- fluoridation against tooth decay: fluoridation to fight tooth decay
- for humans or mammals: for humans or other mammals
- Support on prose per standard disclaimer, but the repetition of "US$" seems counter to WP:$. These are my edits. - Dank (push to talk) 02:47, 8 August 2014 (UTC)[reply]
Closing note - The remaining issues appear to be minor and these can be attended to post FAC. Please attend to these a.s.a.p, (the query about reference 55 for example).
- As for me, I am unfortunately unable do that at the very moment, but (of course) will do that as soon as I'm able to do that (can sit in front of a computer connected to the Internet), in a week or so. Not much longer.--R8R (talk) 20:05, 15 August 2014 (UTC)[reply]
- @R8R Gtrs: I believe that source #55 can be found in Emsley 2011 (I returned that book to the library already). Parcly Taxel 11:05, 16 August 2014 (UTC)[reply]
- @R8R Gtrs: No wait, Ullmann has it, #55 patched. Parcly Taxel 11:45, 16 August 2014 (UTC)[reply]
- @R8R Gtrs: I believe that source #55 can be found in Emsley 2011 (I returned that book to the library already). Parcly Taxel 11:05, 16 August 2014 (UTC)[reply]
- Closing note: This candidate has been promoted, but there may be a delay in bot processing of the close. Please see WP:FAC/ar, and leave the ((featured article candidates)) template in place on the talk page until the bot goes through. Graham Colm (talk) 09:17, 15 August 2014 (UTC)[reply]
- The above discussion is preserved as an archive. Please do not modify it. No further edits should be made to this page.