
Pyrolysis is the process of thermal decomposition of materials at elevated temperatures, often in an inert atmosphere.[1]
Etymology
[edit]The word pyrolysis is coined from the Greek-derived elements pyro- (from Ancient Greek πῦρ : pûr - "fire, heat, fever") and lysis (λύσις : lúsis - "separation, loosening").
Applications
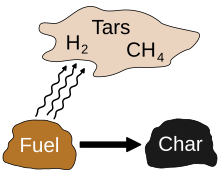
[edit]Pyrolysis is most commonly used in the treatment of organic materials. It is one of the processes involved in charring wood.[2] In general, pyrolysis of organic substances produces volatile products and leaves char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly carbon as the residue, is called carbonization. Pyrolysis is considered the first step in the processes of gasification or combustion.[3][4]
The process is used heavily in the chemical industry, for example, to produce ethylene, many forms of carbon, and other chemicals from petroleum, coal, and even wood, or to produce coke from coal. It is used also in the conversion of natural gas (primarily methane) into hydrogen gas and solid carbon char, recently introduced on an industrial scale.[5] Aspirational applications of pyrolysis would convert biomass into syngas and biochar, waste plastics back into usable oil, or waste into safely disposable substances.
Terminology
[edit]Pyrolysis is one of the various types of chemical degradation processes that occur at higher temperatures (above the boiling point of water or other solvents). It differs from other processes like combustion and hydrolysis in that it usually does not involve the addition of other reagents such as oxygen (O2, in combustion) or water (in hydrolysis).[6] Pyrolysis produces solids (char), condensable liquids, (light and heavy oils and tar), and non-condensable gasses.[7][8][9][10]
Pyrolysis is different from gasification. In the chemical process industry, pyrolysis refers to a partial thermal degradation of carbonaceous materials that takes place in an inert (oxygen free) atmosphere and produces both gases, liquids and solids. The pyrolysis can be extended to full gasification that produces mainly gaseous output,[11] often with the addition of e.g. water steam to gasify residual carbonic solids, see Steam reforming.
Types
[edit]Specific types of pyrolysis include:
- Carbonization, the complete pyrolysis of organic matter, which usually leaves a solid residue that consists mostly of elemental carbon.
- Methane pyrolysis, the direct conversion of methane to hydrogen fuel and separable solid carbon, sometimes using molten metal catalysts.
- Hydrous pyrolysis, in the presence of superheated water or steam, producing hydrogen and substantial atmospheric carbon dioxide.
- Dry distillation, as in the original production of sulfuric acid from sulfates.
- Destructive distillation, as in the manufacture of charcoal, coke and activated carbon.
- Charcoal burning, the production of charcoal.
- Tar production by destructive distillation of wood in tar kilns.
- Caramelization of sugars.
- High-temperature cooking processes such as roasting, frying, toasting, and grilling.
- Cracking of heavier hydrocarbons into lighter ones, as in oil refining.
- Thermal depolymerization, which breaks down plastics and other polymers into monomers and oligomers.
- Ceramization[12] involving the formation of polymer derived ceramics from preceramic polymers under an inert atmosphere.
- Catagenesis, the natural conversion of buried organic matter to fossil fuels.
- Flash vacuum pyrolysis, used in organic synthesis.
Other pyrolysis types come from a different classification that focuses on the pyrolysis operating conditions and heating system used, which have an impact on the yield of the pyrolysis products.
| Pyrolysis | Operating conditions | Pyrolysis product yield (wt%) |
| Slow pyrolysis [13] | Temperature: 300-700 °C
Vapor residence time: 10-100 min Heating rate: 0.1-1 °C/s Feedstock size: 5-50 mm |
Bio-oil ~30
Biochar~35 Gases~35 |
| Intermediate pyrolysis[14] | Temperature: 500-650 °C
Vapor residence time: 0.5-20 s Heating rate: 1.0-10 °C/s Feedstock size: 1-5 mm |
Bio-oil~50
Biochar~25 Gases~35 |
| Fast pyrolysis[13] | Temperature: 400-800 °C
Vapor residence time: 0.5-5 s Heating rate: 10-200 °C/s Feedstock size: <3 mm |
Bio-oil ~50
Biochar~20 Gases~30 |
| Flash pyrolysis[13] | Temperature: 800-1000 °C
Vapor residence time: <5 s Heating rate: >1000 °C/s Feedstock size: <0.2 mm |
Bio-oil ~75
Biochar~12 Gases~13 |
| Hydro pyrolysis[14] | Temperature: 350-600 °C
Vapor residence time: >15 s Heating rate: 10-300 °C/s |
Not assigned |
History
[edit]
Pyrolysis has been used for turning wood into charcoal since ancient times. The ancient Egyptians used the liquid fraction obtained from the pyrolysis of cedar wood, in their embalming process.[15]
The dry distillation of wood remained the major source of methanol into the early 20th century.[16] Pyrolysis was instrumental in the discovery of many chemical substances, such as phosphorus from ammonium sodium hydrogen phosphate NH4NaHPO4 in concentrated urine, oxygen from mercuric oxide, and various nitrates.[citation needed]
General processes and mechanisms
[edit]
Pyrolysis generally consists in heating the material above its decomposition temperature, breaking chemical bonds in its molecules. The fragments usually become smaller molecules, but may combine to produce residues with larger molecular mass, even amorphous covalent solids.[citation needed]
In many settings, some amounts of oxygen, water, or other substances may be present, so that combustion, hydrolysis, or other chemical processes may occur besides pyrolysis proper. Sometimes those chemicals are added intentionally, as in the burning of firewood, in the traditional manufacture of charcoal, and in the steam cracking of crude oil.[citation needed]
Conversely, the starting material may be heated in a vacuum or in an inert atmosphere to avoid chemical side reactions (such as combustion or hydrolysis). Pyrolysis in a vacuum also lowers the boiling point of the byproducts, improving their recovery.
When organic matter is heated at increasing temperatures in open containers, the following processes generally occur, in successive or overlapping stages:[citation needed]
- Below about 100 °C, volatiles, including some water, evaporate. Heat-sensitive substances, such as vitamin C and proteins, may partially change or decompose already at this stage.
- At about 100 °C or slightly higher, any remaining water that is merely absorbed in the material is driven off. This process consumes a lot of energy, so the temperature may stop rising until all water has evaporated. Water trapped in crystal structure of hydrates may come off at somewhat higher temperatures.
- Some solid substances, like fats, waxes, and sugars, may melt and separate.
- Between 100 and 500 °C, many common organic molecules break down. Most sugars start decomposing at 160–180 °C. Cellulose, a major component of wood, paper, and cotton fabrics, decomposes at about 350 °C.[3] Lignin, another major wood component, starts decomposing at about 350 °C, but continues releasing volatile products up to 500 °C.[3] The decomposition products usually include water, carbon monoxide CO and/or carbon dioxide CO2, as well as a large number of organic compounds.[4][17] Gases and volatile products leave the sample, and some of them may condense again as smoke. Generally, this process also absorbs energy. Some volatiles may ignite and burn, creating a visible flame. The non-volatile residues typically become richer in carbon and form large disordered molecules, with colors ranging between brown and black. At this point the matter is said to have been "charred" or "carbonized".
- At 200–300 °C, if oxygen has not been excluded, the carbonaceous residue may start to burn, in a highly exothermic reaction, often with no or little visible flame. Once carbon combustion starts, the temperature rises spontaneously, turning the residue into a glowing ember and releasing carbon dioxide and/or monoxide. At this stage, some of the nitrogen still remaining in the residue may be oxidized into nitrogen oxides like NO2 and N2O3. Sulfur and other elements like chlorine and arsenic may be oxidized and volatilized at this stage.
- Once combustion of the carbonaceous residue is complete, a powdery or solid mineral residue (ash) is often left behind, consisting of inorganic oxidized materials of high melting point. Some of the ash may have left during combustion, entrained by the gases as fly ash or particulate emissions. Metals present in the original matter usually remain in the ash as oxides or carbonates, such as potash. Phosphorus, from materials such as bone, phospholipids, and nucleic acids, usually remains as phosphates.
Safety challenges
[edit]Because pyrolysis takes place at high temperatures which exceed the autoignition temperature of the produced gases, an explosion risk exists if oxygen is present. To control the temperature of pyrolysis systems careful temperature control is needed and can be accomplished with an open source pyrolysis controller.[18] Pyrolysis also produces various toxic gases, mainly carbon monoxide. The greatest risk of fire, explosion and release of toxic gases comes when the system is starting up and shutting down, operating intermittently, or during operational upsets.[19]
Inert gas purging is essential to manage inherent explosion risks. The procedure is not trivial and failure to keep oxygen out has led to accidents.[20]
Occurrence and uses
[edit]Cooking
[edit]Pyrolysis has many applications in food preparation.[21] Caramelization is the pyrolysis of sugars in food (often after the sugars have been produced by the breakdown of polysaccharides). The food goes brown and changes flavor. The distinctive flavors are used in many dishes; for instance, caramelized onion is used in French onion soup.[22][23] The temperatures needed for caramelization lie above the boiling point of water.[22] Frying oil can easily rise above the boiling point. Putting a lid on the frying pan keeps the water in, and some of it re-condenses, keeping the temperature too cool to brown for longer time.
Pyrolysis of food can also be undesirable, as in the charring of burnt food (at temperatures too low for the oxidative combustion of carbon to produce flames and burn the food to ash).
Coke, carbon, charcoals, and chars
[edit]Carbon and carbon-rich materials have desirable properties but are nonvolatile, even at high temperatures. Consequently, pyrolysis is used to produce many kinds of carbon; these can be used for fuel, as reagents in steelmaking (coke), and as structural materials.
Charcoal is a less smoky fuel than pyrolyzed wood.[24] Some cities ban, or used to ban, wood fires; when residents only use charcoal (and similarly treated rock coal, called coke) air pollution is significantly reduced. In cities where people do not generally cook or heat with fires, this is not needed. In the mid-20th century, "smokeless" legislation in Europe required cleaner-burning techniques, such as coke fuel[25] and smoke-burning incinerators[26] as an effective measure to reduce air pollution[25]


The coke-making or "coking" process consists of heating the material in "coking ovens" to very high temperatures (up to 900 °C or 1,700 °F) so that the molecules are broken down into lighter volatile substances, which leave the vessel, and a porous but hard residue that is mostly carbon and inorganic ash. The amount of volatiles varies with the source material, but is typically 25–30% of it by weight. High temperature pyrolysis is used on an industrial scale to convert coal into coke. This is useful in metallurgy, where the higher temperatures are necessary for many processes, such as steelmaking. Volatile by-products of this process are also often useful, including benzene and pyridine.[27] Coke can also be produced from the solid residue left from petroleum refining.
The original vascular structure of the wood and the pores created by escaping gases combine to produce a light and porous material. By starting with a dense wood-like material, such as nutshells or peach stones, one obtains a form of charcoal with particularly fine pores (and hence a much larger pore surface area), called activated carbon, which is used as an adsorbent for a wide range of chemical substances.
Biochar is the residue of incomplete organic pyrolysis, e.g., from cooking fires. It is a key component of the terra preta soils associated with ancient indigenous communities of the Amazon basin.[28] Terra preta is much sought by local farmers for its superior fertility and capacity to promote and retain an enhanced suite of beneficial microbiota, compared to the typical red soil of the region. Efforts are underway to recreate these soils through biochar, the solid residue of pyrolysis of various materials, mostly organic waste.

Carbon fibers are filaments of carbon that can be used to make very strong yarns and textiles. Carbon fiber items are often produced by spinning and weaving the desired item from fibers of a suitable polymer, and then pyrolyzing the material at a high temperature (from 1,500–3,000 °C or 2,730–5,430 °F). The first carbon fibers were made from rayon, but polyacrylonitrile has become the most common starting material. For their first workable electric lamps, Joseph Wilson Swan and Thomas Edison used carbon filaments made by pyrolysis of cotton yarns and bamboo splinters, respectively.
Pyrolysis is the reaction used to coat a preformed substrate with a layer of pyrolytic carbon. This is typically done in a fluidized bed reactor heated to 1,000–2,000 °C or 1,830–3,630 °F. Pyrolytic carbon coatings are used in many applications, including artificial heart valves.[29]
Liquid and gaseous biofuels
[edit]Pyrolysis is the basis of several methods for producing fuel from biomass, i.e. lignocellulosic biomass.[30] Crops studied as biomass feedstock for pyrolysis include native North American prairie grasses such as switchgrass and bred versions of other grasses such as Miscantheus giganteus. Other sources of organic matter as feedstock for pyrolysis include greenwaste, sawdust, waste wood, leaves, vegetables, nut shells, straw, cotton trash, rice hulls, and orange peels.[3] Animal waste including poultry litter, dairy manure, and potentially other manures are also under evaluation. Some industrial byproducts are also suitable feedstock including paper sludge, distillers grain,[31] and sewage sludge.[32]
In the biomass components, the pyrolysis of hemicellulose happens between 210 and 310 °C.[3] The pyrolysis of cellulose starts from 300 to 315 °C and ends at 360–380 °C, with a peak at 342–354 °C.[3] Lignin starts to decompose at about 200 °C and continues until 1000 °C.[33]
Synthetic diesel fuel by pyrolysis of organic materials is not yet economically competitive.[34] Higher efficiency is sometimes achieved by flash pyrolysis, in which finely divided feedstock is quickly heated to between 350 and 500 °C (660 and 930 °F) for less than two seconds.
Syngas is usually produced by pyrolysis.[21]
The low quality of oils produced through pyrolysis can be improved by physical and chemical processes,[35] which might drive up production costs, but may make sense economically as circumstances change.
There is also the possibility of integrating with other processes such as mechanical biological treatment and anaerobic digestion.[36] Fast pyrolysis is also investigated for biomass conversion.[37] Fuel bio-oil can also be produced by hydrous pyrolysis.
Methane pyrolysis for hydrogen
[edit]
Methane pyrolysis[38] is an industrial process for "turquoise" hydrogen production from methane by removing solid carbon from natural gas.[39] This one-step process produces hydrogen in high volume at low cost (less than steam reforming with carbon sequestration).[40] No greenhouse gas is released. No deep well injection of carbon dioxide is needed. Only water is released when hydrogen is used as the fuel for fuel-cell electric heavy truck transportation, [41][42][43][44][45] gas turbine electric power generation,[46][47] and hydrogen for industrial processes including producing ammonia fertilizer and cement.[48][49] Methane pyrolysis is the process operating around 1065 °C for producing hydrogen from natural gas that allows removal of carbon easily (solid carbon is a byproduct of the process).[50][51] The industrial quality solid carbon can then be sold or landfilled and is not released into the atmosphere, avoiding emission of greenhouse gas (GHG) or ground water pollution from a landfill. In 2015, a company called Monolith Materials built a pilot plant in Redwood City, CA to study scaling Methane Pyrolysis using renewable power in the process.[52] A successful pilot project then led to a larger commercial-scale demonstration plant in Hallam, Nebraska in 2016.[53] As of 2020, this plant is operational and can produce around 14 metric tons of hydrogen per day. In 2021, the US Department of Energy backed Monolith Materials' plans for major expansion with a $1B loan guarantee.[54] The funding will help produce a plant capable of generating 164 metric tons of hydrogen per day by 2024. Pilots with gas utilities and biogas plants are underway with companies like Modern Hydrogen.[55][56] Volume production is also being evaluated in the BASF "methane pyrolysis at scale" pilot plant,[5] the chemical engineering team at University of California - Santa Barbara[57] and in such research laboratories as Karlsruhe Liquid-metal Laboratory (KALLA).[58] Power for process heat consumed is only one-seventh of the power consumed in the water electrolysis method for producing hydrogen.[59]
The Australian company Hazer Group was founded in 2010 to commercialise technology originally developed at the University of Western Australia. The company was listed on the ASX in December 2015. It is completing a commercial demonstration project to produce renewable hydrogen and graphite from wastewater and iron ore as a process catalyst use technology created by the University of Western Australia (UWA). The Commercial Demonstration Plant project is an Australian first, and expected to produce around 100 tonnes of fuel-grade hydrogen and 380 tonnes of graphite each year starting in 2023.[60] It was scheduled to commence in 2022. "10 December 2021: Hazer Group (ASX: HZR) regret to advise that there has been a delay to the completion of the fabrication of the reactor for the Hazer Commercial Demonstration Project (CDP). This is expected to delay the planned commissioning of the Hazer CDP, with commissioning now expected to occur after our current target date of 1Q 2022."[61] The Hazer Group has collaboration agreements with Engie for a facility in France in May 2023,[62] A Memorandum of Understanding with Chubu Electric & Chiyoda in Japan April 2023[63] and an agreement with Suncor Energy and FortisBC to develop 2,500 tonnes per Annum Burrard-Hazer Hydrogen Production Plant in Canada April 2022[64][65]
The American company C-Zero's technology converts natural gas into hydrogen and solid carbon. The hydrogen provides clean, low-cost energy on demand, while the carbon can be permanently sequestered.[66] C-Zero announced in June 2022 that it closed a $34 million financing round led by SK Gas, a subsidiary of South Korea's second-largest conglomerate, the SK Group. SK Gas was joined by two other new investors, Engie New Ventures and Trafigura, one of the world's largest physical commodities trading companies, in addition to participation from existing investors including Breakthrough Energy Ventures, Eni Next, Mitsubishi Heavy Industries, and AP Ventures. Funding was for C-Zero's first pilot plant, which was expected to be online in Q1 2023. The plant may be capable of producing up to 400 kg of hydrogen per day from natural gas with no CO2 emissions.[67]
One of the world's largest chemical companies, BASF, has been researching hydrogen pyrolysis for more than 10 years.[68]
Ethylene
[edit]Pyrolysis is used to produce ethylene, the chemical compound produced on the largest scale industrially (>110 million tons/year in 2005). In this process, hydrocarbons from petroleum are heated to around 600 °C (1,112 °F) in the presence of steam; this is called steam cracking. The resulting ethylene is used to make antifreeze (ethylene glycol), PVC (via vinyl chloride), and many other polymers, such as polyethylene and polystyrene.[69]
Semiconductors
[edit]
The process of metalorganic vapour-phase epitaxy (MOCVD) entails pyrolysis of volatile organometallic compounds to give semiconductors, hard coatings, and other applicable materials. The reactions entail thermal degradation of precursors, with deposition of the inorganic component and release of the hydrocarbons as gaseous waste. Since it is an atom-by-atom deposition, these atoms organize themselves into crystals to form the bulk semiconductor. Raw polycrystalline silicon is produced by the chemical vapor deposition of silane gases:
- SiH4 → Si + 2 H2.
Gallium arsenide, another semiconductor, forms upon co-pyrolysis of trimethylgallium and arsine.
Waste management
[edit]Pyrolysis can also be used to treat municipal solid waste and plastic waste.[4][17][70] The main advantage is the reduction in volume of the waste. In principle, pyrolysis will regenerate the monomers (precursors) to the polymers that are treated, but in practice the process is neither a clean nor an economically competitive source of monomers.[71][72][73]
In tire waste management, tire pyrolysis is a well-developed technology.[74] Other products from car tire pyrolysis include steel wires, carbon black and bitumen.[75] The area faces legislative, economic, and marketing obstacles.[76] Oil derived from tire rubber pyrolysis has a high sulfur content, which gives it high potential as a pollutant; consequently it should be desulfurized.[77][78]
Alkaline pyrolysis of sewage sludge at low temperature of 500 °C can enhance H2 production with in-situ carbon capture. The use of NaOH (sodium hydroxide) has the potential to produce H2-rich gas that can be used for fuels cells directly.[32][79]
In early November 2021, the U.S. State of Georgia announced a joint effort with Igneo Technologies to build an $85 million large electronics recycling plant in the Port of Savannah. The project will focus on lower-value, plastics-heavy devices in the waste stream using multiple shredders and furnaces using pyrolysis technology.[80]
One-stepwise pyrolysis and Two-stepwise pyrolysis for Tobacco Waste
[edit]Pyrolysis has also been used for trying to mitigate tobacco waste. One method was done where tobacco waste was separated into two categories TLW (Tobacco Leaf Waste) and TSW (Tobacco Stick Waste). TLW was determined to be any waste from cigarettes and TSW was determined to be any waste from electronic cigarettes. Both TLW and TSW were dried at 80 °C for 24 hours and stored in a desiccator.[81] Samples were grounded so that the contents were uniform. Tobacco Waste (TW) also contains inorganic (metal) contents, which was determined using an inductively coupled plasma-optical spectrometer.[81] Thermo-gravimetric analysis was used to thermally degrade four samples (TLW, TSW, glycerol, and guar gum) and monitored under specific dynamic temperature conditions.[81] About one gram of both TLW and TSW were used in the pyrolysis tests. During these analysis tests, CO2 and N2 were used as atmospheres inside of a tubular reactor that was built using quartz tubing. For both CO2 and N2 atmospheres the flow rate was 100 mL min−1.[81] External heating was created via a tubular furnace. The pyrogenic products were classified into three phases. The first phase was biochar, a solid residue produced by the reactor at 650 °C. The second phase liquid hydrocarbons were collected by a cold solvent trap and sorted by using chromatography. The third and final phase was analyzed using an online micro GC unit and those pyrolysates were gases.
Two different types of experiments were conducted: one-stepwise pyrolysis and two-stepwise pyrolysis. One-stepwise pyrolysis consisted of a constant heating rate (10 °C min−1) from 30 to 720 °C.[81] In the second step of the two-stepwise pyrolysis test the pyrolysates from the one-stepwise pyrolysis were pyrolyzed in the second heating zone which was controlled isothermally at 650 °C.[81] The two-stepwise pyrolysis was used to focus primarily on how well CO2 affects carbon redistribution when adding heat through the second heating zone.[81]
First noted was the thermolytic behaviors of TLW and TSW in both the CO2 and N2 environments. For both TLW and TSW the thermolytic behaviors were identical at less than or equal to 660 °C in the CO2 and N2 environments. The differences between the environments start to occur when temperatures increase above 660 °C and the residual mass percentages significantly decrease in the CO2 environment compared to that in the N2 environment.[81] This observation is likely due to the Boudouard reaction, where we see spontaneous gasification happening when temperatures exceed 710 °C.[82][83] Although these observations were seen at temperatures lower than 710 °C it is most likely due to the catalytic capabilities of inorganics in TLW.[81] It was further investigated by doing ICP-OES measurements and found that a fifth of the residual mass percentage was Ca species. CaCO3 is used in cigarette papers and filter material, leading to the explanation that degradation of CaCO3 causes pure CO2 reacting with CaO in a dynamic equilibrium state.[81] This being the reason for seeing mass decay between 660 °C and 710 °C. Differences in differential thermogram (DTG) peaks for TLW were compared to TSW. TLW had four distinctive peaks at 87, 195, 265, and 306 °C whereas TSW had two major drop offs at 200 and 306 °C with one spike in between.[81] The four peaks indicated that TLW contains more diverse types of additives than TSW.[81] The residual mass percentage between TLW and TSW was further compared, where the residual mass in TSW was less than that of TLW for both CO2 and N2 environments concluding that TSW has higher quantities of additives than TLW.
The one-stepwise pyrolysis experiment showed different results for the CO2 and N2 environments. During this process the evolution of 5 different notable gases were observed. Hydrogen, Methane, Ethane, Carbon Dioxide, and Ethylene all are produced when the thermolytic rate of TLW began to be retarded at greater than or equal to 500 °C. Thermolytic rate begins at the same temperatures for both the CO2 and N2 environment but there is higher concentration of the production of Hydrogen, Ethane, Ethylene, and Methane in the N2 environment than that in the CO2 environment. The concentration of CO in the CO2 environment is significantly greater as temperatures increase past 600 °C and this is due to CO2 being liberated from CaCO3 in TLW.[81] This significant increase in CO concentration is why there is lower concentrations of other gases produced in the CO2 environment due to a dilution effect.[81] Since pyrolysis is the re-distribution of carbons in carbon substrates into three pyrogenic products.[81] The CO2 environment is going to be more effective because the CO2 reduction into CO allows for the oxidation of pyrolysates to form CO. In conclusion the CO2 environment allows a higher yield of gases than oil and biochar. When the same process is done for TSW the trends are almost identical therefore the same explanations can be applied to the pyrolysis of TSW.[81]
Harmful chemicals were reduced in the CO2 environment due to CO formation causing tar to be reduced. One-stepwise pyrolysis was not that effective on activating CO2 on carbon rearrangement due to the high quantities of liquid pyrolysates (tar). Two-stepwise pyrolysis for the CO2 environment allowed for greater concentrations of gases due to the second heating zone. The second heating zone was at a consistent temperature of 650 °C isothermally.[81] More reactions between CO2 and gaseous pyrolysates with longer residence time meant that CO2 could further convert pyrolysates into CO.[81] The results showed that the two-stepwise pyrolysis was an effective way to decrease tar content and increase gas concentration by about 10 wt.% for both TLW (64.20 wt.%) and TSW (73.71%).[81]
Thermal cleaning
[edit]Pyrolysis is also used for thermal cleaning, an industrial application to remove organic substances such as polymers, plastics and coatings from parts, products or production components like extruder screws, spinnerets[84] and static mixers. During the thermal cleaning process, at temperatures from 310 to 540 °C (600 to 1,000 °F),[85] organic material is converted by pyrolysis and oxidation into volatile organic compounds, hydrocarbons and carbonized gas.[86] Inorganic elements remain.[87]
Several types of thermal cleaning systems use pyrolysis:
- Molten Salt Baths belong to the oldest thermal cleaning systems; cleaning with a molten salt bath is very fast but implies the risk of dangerous splatters, or other potential hazards connected with the use of salt baths, like explosions or highly toxic hydrogen cyanide gas.[85]
- Fluidized Bed Systems[88] use sand or aluminium oxide as heating medium;[89] these systems also clean very fast but the medium does not melt or boil, nor emit any vapors or odors;[85] the cleaning process takes one to two hours.[86]
- Vacuum Ovens use pyrolysis in a vacuum[90] avoiding uncontrolled combustion inside the cleaning chamber;[85] the cleaning process takes 8[86] to 30 hours.[91]
- Burn-Off Ovens, also known as Heat-Cleaning Ovens, are gas-fired and used in the painting, coatings, electric motors and plastics industries for removing organics from heavy and large metal parts.[92]
Fine chemical synthesis
[edit]Pyrolysis is used in the production of chemical compounds, mainly, but not only, in the research laboratory.
The area of boron-hydride clusters started with the study of the pyrolysis of diborane (B2H6) at ca. 200 °C. Products include the clusters pentaborane and decaborane. These pyrolyses involve not only cracking (to give H2), but also recondensation.[93]
The synthesis of nanoparticles,[94] zirconia[95] and oxides[96] utilizing an ultrasonic nozzle in a process called ultrasonic spray pyrolysis (USP).
Other uses and occurrences
[edit]- Pyrolysis is used to turn organic materials into carbon for the purpose of carbon-14 dating.
- Pyrolysis liquids from slow pyrolysis of bark and hemp have been tested for their antifungal activity against wood decaying fungi, showing potential to substitute the current wood preservatives[97] while further tests are still required. However, their ecotoxicity is very variable and while some are less toxic than current wood preservatives, other pyrolysis liquids have shown high ecotoxicity, what may cause detrimental effects in the environment.[98]
- Pyrolysis of tobacco, paper, and additives, in cigarettes and other products, generates many volatile products (including nicotine, carbon monoxide, and tar) that are responsible for the aroma and negative health effects of smoking. Similar considerations apply to the smoking of marijuana and the burning of incense products and mosquito coils.
- Pyrolysis occurs during the incineration of trash, potentially generating volatiles that are toxic or contribute to air pollution if not completely burned.
- Laboratory or industrial equipment sometimes gets fouled by carbonaceous residues that result from coking, the pyrolysis of organic products that come into contact with hot surfaces.
PAHs generation
[edit]Polycyclic aromatic hydrocarbons (PAHs) can be generated from the pyrolysis of different solid waste fractions,[10] such as hemicellulose, cellulose, lignin, pectin, starch, polyethylene (PE), polystyrene (PS), polyvinyl chloride (PVC), and polyethylene terephthalate (PET). PS, PVC, and lignin generate significant amount of PAHs. Naphthalene is the most abundant PAH among all the polycyclic aromatic hydrocarbons.[99]
When the temperature is increased from 500 to 900 °C, most PAHs increase. With increasing temperature, the percentage of light PAHs decreases and the percentage of heavy PAHs increases.[100][101]
Study tools
[edit]Thermogravimetric analysis
[edit]Thermogravimetric analysis (TGA) is one of the most common techniques to investigate pyrolysis with no limitations of heat and mass transfer. The results can be used to determine mass loss kinetics.[3][17][4][33][70] Activation energies can be calculated using the Kissinger method or peak analysis-least square method (PA-LSM).[4][33]
TGA can couple with Fourier-transform infrared spectroscopy (FTIR) and mass spectrometry. As the temperature increases, the volatiles generated from pyrolysis can be measured.[102][79]
Macro-TGA
[edit]In TGA, the sample is loaded first before the increase of temperature, and the heating rate is low (less than 100 °C min−1). Macro-TGA can use gram-scale samples, which can be used to investigate the pyrolysis with mass and heat transfer effects.[4][103]
Pyrolysis–gas chromatography–mass spectrometry
[edit]Pyrolysis mass spectrometry (Py-GC-MS) is an important laboratory procedure to determine the structure of compounds.[104][105]
Machine learning
[edit]In recent years, machine learning has attracted significant research interest in predicting yields, optimizing parameters, and monitoring pyrolytic processes.[106][107]
See also
[edit]References
[edit]- ^ "Pyrolysis". Compendium of Chemical Terminology. International Union of Pure and Applied Chemistry. 2009. p. 1824. doi:10.1351/goldbook.P04961. ISBN 978-0-9678550-9-7. Retrieved 2018-01-10.
- ^ "Burning of wood". InnoFireWood's website. Archived from the original on 2010-02-09. Retrieved 2010-02-06.
- ^ a b c d e f g Zhou, Hui; Long, YanQiu; Meng, AiHong; Li, QingHai; Zhang, YanGuo (August 2013). "The pyrolysis simulation of five biomass species by hemi-cellulose, cellulose and lignin based on thermogravimetric curves". Thermochimica Acta. 566: 36–43. Bibcode:2013TcAc..566...36Z. doi:10.1016/j.tca.2013.04.040.
- ^ a b c d e f Zhou, Hui (2017). "Combustible Solid Waste Thermochemical Conversion". Springer Theses. doi:10.1007/978-981-10-3827-3. ISBN 978-981-10-3826-6. ISSN 2190-5053. S2CID 135947379.
- ^ a b BASF. "BASF researchers working on fundamentally new, low-carbon production processes, Methane Pyrolysis". United States Sustainability. BASF. Archived from the original on 19 October 2020. Retrieved 19 October 2020.
- ^ Cory A. Kramer, Reza Loloee, Indrek S. Wichman and Ruby N. Ghosh, 2009, Time Resolved Measurements of Pyrolysis Products From Thermoplastic Poly-Methyl-Methacrylate (PMMA) Archived 2014-11-06 at the Wayback Machine ASME 2009 International Mechanical Engineering Congress and Exposition
- ^ Ramin, L.; Assadi, M. Hussein N.; Sahajwalla, V. (2014). "High-density polyethylene degradation into low molecular weight gases at 1823K: An atomistic simulation". J. Anal. Appl. Pyrol. 110: 318–321. arXiv:2204.08253. Bibcode:2014JAAP..110..318R. doi:10.1016/j.jaap.2014.09.022. S2CID 96961784.
- ^ Jones, Jim. "Mechanisms of pyrolysis" (PDF). Retrieved 19 May 2019.
- ^ George, Anthe; Turn, Scott Q.; Morgan, Trevor James (26 August 2015). "Fast Pyrolysis Behavior of Banagrass as a Function of Temperature and Volatiles Residence Time in a Fluidized Bed Reactor". PLOS ONE. 10 (8): e0136511. Bibcode:2015PLoSO..1036511M. doi:10.1371/journal.pone.0136511. ISSN 1932-6203. PMC 4550300. PMID 26308860.
- ^ a b Zhou, Hui; Wu, Chunfei; Meng, Aihong; Zhang, Yanguo; Williams, Paul T. (November 2014). "Effect of interactions of biomass constituents on polycyclic aromatic hydrocarbons (PAH) formation during fast pyrolysis" (PDF). Journal of Analytical and Applied Pyrolysis. 110: 264–269. Bibcode:2014JAAP..110..264Z. doi:10.1016/j.jaap.2014.09.007.
- ^ Astrup, T., & Bilitewski, B. (2011). Pyrolysis and Gasification. In Solid Waste Technology and Management (Vol. Volume 1. Chapter 8.8, pp. 502-512). Wiley.
- ^ Wang, Xifan; Schmidt, Franziska; Hanaor, Dorian; Kamm, Paul H.; Li, Shuang; Gurlo, Aleksander (2019). "Additive manufacturing of ceramics from preceramic polymers: A versatile stereolithographic approach assisted by thiol-ene click chemistry". Additive Manufacturing. 27: 80–90. arXiv:1905.02060. Bibcode:2019arXiv190502060W. doi:10.1016/j.addma.2019.02.012. S2CID 104470679.
- ^ a b c Jenkins, R. W.; Sutton, A. D.; Robichaud, D. J. (2016-01-01), Chuck, Christopher J. (ed.), "Chapter 8 - Pyrolysis of Biomass for Aviation Fuel", Biofuels for Aviation, Academic Press, pp. 191–215, ISBN 978-0-12-804568-8, retrieved 2023-12-12
- ^ a b Tripathi, Manoj; Sahu, J. N.; Ganesan, P. (2016-03-01). "Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review". Renewable and Sustainable Energy Reviews. 55: 467–481. Bibcode:2016RSERv..55..467T. doi:10.1016/j.rser.2015.10.122. ISSN 1364-0321.
- ^ Koller, Johann; Baumer, Ursula; Kaup, Yoka; Schmid, Mirjam; Weser, Ulrich (October 2003). "Analysis of a pharaonic embalming tar". Nature. 425 (6960): 784. doi:10.1038/425784a. ISSN 1476-4687. PMID 14574400.
- ^ E. Fiedler; G. Grossmann; D. B. Kersebohm; G. Weiss; Claus Witte (2005). "Methanol". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007. ISBN 978-3-527-30673-2.
- ^ a b c Zhou, Hui; Long, YanQiu; Meng, AiHong; Li, QingHai; Zhang, YanGuo (April 2015). "Thermogravimetric characteristics of typical municipal solid waste fractions during co-pyrolysis". Waste Management. 38: 194–200. Bibcode:2015WaMan..38..194Z. doi:10.1016/j.wasman.2014.09.027. PMID 25680236.
- ^ Hafting, Finn K.; Kulas, Daniel; Michels, Etienne; Chipkar, Sarvada; Wisniewski, Stefan; Shonnard, David; Pearce, Joshua M. (2023). "Modular Open-Source Design of Pyrolysis Reactor Monitoring and Control Electronics". Electronics. 12 (24): 4893. doi:10.3390/electronics12244893. ISSN 2079-9292.
- ^ Rollinson, A. N. (2018) 'Fire, explosion and chemical toxicity hazards of gasification energy from waste', Journal of Loss Prevention in the Process Industries, 54, pp. 273–280. doi:10.1016/j.jlp.2018.04.010.
- ^ Hedlund F.H., 2023, Inherent Hazards and Limited Regulatory Oversight in the Waste Plastic Recycling Sector – Repeat Explosion at Pyrolysis Plant, Chemical Engineering Transactions, 99, 241-246 DOI:10.3303/CET2399041
- ^ a b Kaplan, Ryan (Fall 2011). "Pyrolysis: Biochar, Bio-Oil and Syngas from Wastes". users.humboldt.edu. Humboldt University. Archived from the original (Course notes for Environmental Resources Engineering 115) on 3 April 2014. Retrieved 19 May 2019.
- ^ a b "What is Caramelization?". www.scienceofcooking.com. Retrieved 19 May 2019.
- ^ Brimm, Courtney (7 November 2011). "Cooking with Chemistry: What is Caramelization?". Common Sense Science. Retrieved 19 May 2019.
- ^ Sood, A (December 2012). "Indoor fuel exposure and the lung in both developing and developed countries: an update". Clinics in Chest Medicine. 33 (4): 649–65. doi:10.1016/j.ccm.2012.08.003. PMC 3500516. PMID 23153607.
- ^ a b "SMOKELESS zones". British Medical Journal. 2 (4840): 818–20. 10 October 1953. doi:10.1136/bmj.2.4840.818. PMC 2029724. PMID 13082128.
- ^ "Two-stage incinerator, United States Patent 3881430". www.freepatentsonline.com. Retrieved 11 February 2023.
- ^ Ludwig Briesemeister; Andreas Geißler; Stefan Halama; Stephan Herrmann; Ulrich Kleinhans; Markus Steibel; Markus Ulbrich; Alan W. Scaroni; M. Rashid Khan; Semih Eser; Ljubisa R. Radovic (2002). "Coal Pyrolysis". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–44. doi:10.1002/14356007.a07_245.pub2. ISBN 978-3-527-30673-2.
- ^ Lehmann, Johannes. "Biochar: the new frontier". Archived from the original on 2008-06-18. Retrieved 2008-07-10.
- ^ Ratner, Buddy D. (2004). Pyrolytic carbon. In Biomaterials science: an introduction to materials in medicine Archived 2014-06-26 at the Wayback Machine. Academic Press. pp. 171–180. ISBN 0-12-582463-7.
- ^ Evans, G. "Liquid Transport Biofuels – Technology Status Report" Archived September 19, 2008, at the Wayback Machine, "National Non-Food Crops Centre", 14-04-08. Retrieved on 2009-05-05.
- ^ "Biomass Feedstock for Slow Pyrolysis". BEST Pyrolysis, Inc. website. BEST Energies, Inc. Archived from the original on 2012-01-02. Retrieved 2010-07-30.
- ^ a b Zhao, Ming; Wang, Fan; Fan, Yiran; Raheem, Abdul; Zhou, Hui (March 2019). "Low-temperature alkaline pyrolysis of sewage sludge for enhanced H2 production with in-situ carbon capture". International Journal of Hydrogen Energy. 44 (16): 8020–8027. doi:10.1016/j.ijhydene.2019.02.040. S2CID 104385409.
- ^ a b c Zhou, Hui; Long, Yanqiu; Meng, Aihong; Chen, Shen; Li, Qinghai; Zhang, Yanguo (2015). "A novel method for kinetics analysis of pyrolysis of hemicellulose, cellulose, and lignin in TGA and macro-TGA". RSC Advances. 5 (34): 26509–26516. Bibcode:2015RSCAd...526509Z. doi:10.1039/C5RA02715B. ISSN 2046-2069.
- ^ "Pyrolysis and Other Thermal Processing". US DOE. Archived from the original on 2007-08-14.
- ^ Ramirez, Jerome; Brown, Richard; Rainey, Thomas (1 July 2015). "A Review of Hydrothermal Liquefaction Bio-Crude Properties and Prospects for Upgrading to Transportation Fuels". Energies. 8 (7): 6765–6794. doi:10.3390/en8076765.
- ^ Marshall, A. T. & Morris, J. M. (2006) A Watery Solution and Sustainable Energy Parks Archived 2007-09-28 at the Wayback Machine, CIWM Journal, pp. 22–23
- ^ Westerhof, Roel Johannes Maria (2011). Refining fast pyrolysis of biomass. Thermo-Chemical Conversion of Biomass (Thesis). University of Twente. Archived from the original on 2013-06-17. Retrieved 2012-05-30.
- ^ Upham, D. Chester (17 November 2017). "Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon in a single reaction step commercial process (at potentially low-cost). This would provide hydrogen from natural gas, essentially forever". Science. 358 (6365). American Association for Advancement of Science: 917–921. doi:10.1126/science.aao5023. PMID 29146810. S2CID 206663568.
- ^ Timmerberg, Sebastian; Kaltschmitt, Martin; Finkbeiner, Matthias (2020). "Hydrogen and hydrogen-derived fuels through methane decomposition of natural gas – GHG emissions and costs". Energy Conversion and Management: X. 7: 100043. Bibcode:2020ECMX....700043T. doi:10.1016/j.ecmx.2020.100043. hdl:11420/6245. ISSN 2590-1745. S2CID 218919070.
- ^ Lumbers, Brock (20 August 2020). Mathematical Modelling and Simulation of Catalyst Deactivation for the Continuous Thermo-Catalytic Decomposition of Methane (Thesis). Rhine-Waal University of Applied Sciences. pp. 12–13. Retrieved 16 March 2022.
- ^ Fialka, John. "Energy Department Looks to Boost Hydrogen Fuel for Big Trucks". E&E News. Scientific American. Retrieved 7 November 2020.
- ^ CCJ News (13 August 2020). "How fuel cell trucks produce electric power and how they're fueled". CCJ News. Commercial Carrier Journal. Retrieved 19 October 2020.
- ^ Toyota. "Hydrogen Fuel-Cell Class 8 Truck". Hydrogen-Powered Truck Will Offer Heavy-Duty Capability and Clean Emissions. Toyota. Retrieved 19 October 2020.
- ^ Colias, Mike (26 October 2020). "Auto Makers Shift Their Hydrogen Focus to Big Rigs". The Wall Street Journal. Retrieved 26 October 2020.
- ^ Honda. "Honda Fuel-Cell Clarity". Clarity Fuel Cell. Honda. Retrieved 19 October 2020.
- ^ GE Turbines. "Hydrogen fueled power turbines". Hydrogen fueled gas turbines. General Electric. Retrieved 19 October 2020.
- ^ Solar Turbines. "Hydrogen fueled power turbines". Power From Hydrogen Gas For Carbon Reduction. Solar Turbines. Retrieved 19 October 2020.
- ^ Crolius, Stephen H. (27 January 2017). "Methane to Ammonia via Pyrolysis". Ammonia Energy Association. Retrieved 19 October 2020.
- ^ Pérez, Jorge. "CEMEX successfully deploys hydrogen-based ground-breaking cement manufacturing technology". www.cemex.com. CEMEX, S.A.B. de C.V. Retrieved 4 April 2021.
- ^ Cartwright, Jon. "The reaction that would give us clean fossil fuels forever". NewScientist. New Scientist Ltd. Retrieved 30 October 2020.
- ^ Karlsruhe Institute of Technology. "Hydrogen from methane without CO2 emissions". Phys.Org. Retrieved 30 October 2020.
- ^ "Successful Demonstration Program Underpins Monolith Materials' Commercialization Plans - Zeton". Zeton Inc. 2019-05-28. Retrieved 2022-01-05.
- ^ "Monolith". monolith-corp.com. Retrieved 2022-01-05.
- ^ "DOE backs Neb. hydrogen, carbon black project with $1B loan guarantee". www.spglobal.com. Retrieved 2022-01-05.
- ^ "NW Natural to Partner with Modern Electron on Exciting Pilot Project to Turn Methane into Clean Hydrogen and Solid Carbon". The Wall Street Journal. 2022-07-27. ISSN 0099-9660. Retrieved 2022-08-24.
- ^ Stiffler, Lisa (2022-04-26). "Cut the BS: This startup is converting cow manure into clean-burning hydrogen fuel". GeekWire. Retrieved 2022-08-24.
- ^ Fernandez, Sonia. "low-cost, low-emissions technology that can convert methane without forming CO2". Phys-Org. American Institute of Physics. Retrieved 19 October 2020.
- ^ Gusev, Alexander. "KITT/IASS - Producing CO2 Free Hydrogen From Natural Gas For Energy Usage". European Energy Innovation. Institute for Advanced Sustainability Studies. Retrieved 30 October 2020.
- ^ "Methane pyrolysis process uses renewable electricity split CH4 into H2 and carbon-black". December 2020. Retrieved 17 December 2020.
- ^ hazergroup.com.au | Commercialising the Hazer Process
- ^ ASX Market announcements, ASX (10 December 2021). "Delay to Reactor Fabrication". asx.com.au. Retrieved 23 May 2023.
- ^ "Hazer advances ENGIE collaboration for facility in France | hazergroup.com.au". Retrieved 2023-05-23.
- ^ "Hazer Signs MOU with Chubu Electric & Chiyoda | hazergroup.com.au". Retrieved 2023-05-23.
- ^ "Hazer Group – Investor Presentation | hazergroup.com.au". Retrieved 2023-05-23.
- ^ "Burrard Hazer Hydrogen Project Announcement | hazergroup.com.au". Retrieved 2023-05-23.
- ^ "C-Zero | Decarbonizing Natural Gas". C-Zero. Retrieved 2023-05-23.
- ^ "C-Zero Closes $34 Million Financing Round Led by SK Gas to Build Natural Gas Decarbonization Pilot". C-Zero. 2022-06-16. Retrieved 2023-05-23.
- ^ "Interview Andreas Bode". www.basf.com. Retrieved 2023-05-23.
- ^ Zimmermann, Heinz; Walz, Roland (2008). "Ethylene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_045.pub3. ISBN 978-3-527-30673-2.
- ^ a b Zhou, Hui; Long, YanQiu; Meng, AiHong; Li, QingHai; Zhang, YanGuo (January 2015). "Interactions of three municipal solid waste components during co-pyrolysis". Journal of Analytical and Applied Pyrolysis. 111: 265–271. Bibcode:2015JAAP..111..265Z. doi:10.1016/j.jaap.2014.08.017.
- ^ Kaminsky, Walter (2000). "Plastics, Recycling". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_057. ISBN 978-3-527-30673-2.
- ^ N.J. Themelis et al. "Energy and Economic Value of Nonrecyclable Plastics and Municipal Solid Wastes that are Currently Landfilled in the Fifty States" Columbia University Earth Engineering Center Archived 2014-05-08 at the Wayback Machine
- ^ "The Plastic to Oil Machine, A\J – Canada's Environmental Voice". Alternativesjournal.ca. 2016-12-07. Archived from the original on 2015-09-09. Retrieved 2016-12-16.
- ^ ผศ.ดร.ศิริรัตน์ จิตการค้า, "ไพโรไลซิสยางรถยนต์หมดสภาพ : กลไกการผลิตน้ำมันเชื้อเพลิงคุณภาพสูง"วิทยาลัยปิโตรเลียมและปิโตรเคมี จุฬาลงกรณ์มหาวิทยาลัย (in Thai) Jidgarnka, S. "Pyrolysis of Expired Car Tires: Mechanics of Producing High Quality Fuels" Archived 2015-02-20 at the Wayback Machine. Chulalongkorn University Department of Petrochemistry
- ^ Roy, C.; Chaala, A.; Darmstadt, H. (1999). "The vacuum pyrolysis of used tires". Journal of Analytical and Applied Pyrolysis. 51 (1–2): 201–221. doi:10.1016/S0165-2370(99)00017-0.
- ^ Martínez, Juan Daniel; Puy, Neus; Murillo, Ramón; García, Tomás; Navarro, María Victoria; Mastral, Ana Maria (2013). "Waste tyre pyrolysis – A review, Renewable and Sustainable". Energy Reviews. 23: 179–213. doi:10.1016/j.rser.2013.02.038.
- ^ Choi, G.-G.; Jung, S.-H.; Oh, S.-J.; Kim, J.-S. (2014). "Total utilization of waste tire rubber through pyrolysis to obtain oils and CO2 activation of pyrolysis char". Fuel Processing Technology. 123: 57–64. doi:10.1016/j.fuproc.2014.02.007.
- ^ Ringer, M.; Putsche, V.; Scahill, J. (2006) Large-Scale Pyrolysis Oil Production: A Technology Assessment and Economic Analysis Archived 2016-12-30 at the Wayback Machine; NREL/TP-510-37779; National Renewable Energy Laboratory (NREL), Golden, CO.
- ^ a b Zhao, Ming; Memon, Muhammad Zaki; Ji, Guozhao; Yang, Xiaoxiao; Vuppaladadiyam, Arun K.; Song, Yinqiang; Raheem, Abdul; Li, Jinhui; Wang, Wei; Zhou, Hui (April 2020). "Alkali metal bifunctional catalyst-sorbents enabled biomass pyrolysis for enhanced hydrogen production". Renewable Energy. 148: 168–175. Bibcode:2020REne..148..168Z. doi:10.1016/j.renene.2019.12.006. S2CID 213747026.
- ^ Leif, Dan (2021-11-03). "Igneo targets low-grade scrap electronics with $85M plant". resource-recycling.com. Retrieved 2021-11-28.
- ^ a b c d e f g h i j k l m n o p q r s Lee, Taewoo; Jung, Sungyup; Lin, Kun-Yi Andrew; Tsang, Yiu Fai; Kwon, Eilhann E. (2021-01-05). "Mitigation of harmful chemical formation from pyrolysis of tobacco waste using CO2". Journal of Hazardous Materials. 401: 123416. doi:10.1016/j.jhazmat.2020.123416. ISSN 0304-3894. PMID 32763706. S2CID 221073670.
- ^ Lahijani, Pooya; Zainal, Zainal Alimuddin; Mohammadi, Maedeh; Mohamed, Abdul Rahman (2015-01-01). "Conversion of the greenhouse gas CO2 to the fuel gas CO via the Boudouard reaction: A review". Renewable and Sustainable Energy Reviews. 41: 615–632. doi:10.1016/j.rser.2014.08.034. ISSN 1364-0321.
- ^ Hunt, Jacob; Ferrari, Anthony; Lita, Adrian; Crosswhite, Mark; Ashley, Bridgett; Stiegman, A. E. (2013-12-27). "Microwave-Specific Enhancement of the Carbon–Carbon Dioxide (Boudouard) Reaction". The Journal of Physical Chemistry C. 117 (51): 26871–26880. doi:10.1021/jp4076965. ISSN 1932-7447.
- ^ Heffungs, Udo (June 2010). "Effective Spinneret Cleaning". Fiber Journal. Archived from the original on 30 June 2016. Retrieved 19 April 2016.
- ^ a b c d Mainord, Kenneth (September 1994). "Cleaning with Heat: Old Technology with a Bright New Future" (PDF). Pollution Prevention Regional Information Center. The Magazine of Critical Cleaning Technology. Archived (PDF) from the original on 8 December 2015. Retrieved 4 December 2015.
- ^ a b c "A Look at Thermal Cleaning Technology". ThermalProcessing.org. Process Examiner. 14 March 2014. Archived from the original on 8 December 2015. Retrieved 4 December 2015.
- ^ Davis, Gary; Brown, Keith (April 1996). "Cleaning Metal Parts and Tooling" (PDF). Pollution Prevention Regional Information Center. Process Heating. Archived (PDF) from the original on 4 March 2016. Retrieved 4 December 2015.
- ^ Schwing, Ewald; Uhrner, Horst (7 October 1999). "Method for removing polymer deposits which have formed on metal or ceramic machine parts, equipment and tools". Espacenet. European Patent Office. Retrieved 19 April 2016.
- ^ Staffin, Herbert Kenneth; Koelzer, Robert A. (28 November 1974). "Cleaning objects in hot fluidised bed – with neutralisation of resultant acidic gas esp. by alkaline metals cpds". Espacenet. European Patent Office. Retrieved 19 April 2016.
- ^ Dwan, Thomas S. (2 September 1980). "Process for vacuum pyrolysis removal of polymers from various objects". Espacenet. European Patent Office. Retrieved 26 December 2015.
- ^ "Vacuum pyrolysis systems". thermal-cleaning.com. Archived from the original on 15 February 2016. Retrieved 11 February 2016.
- ^ "Paint Stripping: Reducing Waste and Hazardous Material". Minnesota Technical Assistance Program. University of Minnesota. July 2008. Archived from the original on 8 December 2015. Retrieved 4 December 2015.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8. gives Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ^ Pingali, Kalyana C.; Rockstraw, David A.; Deng, Shuguang (2005). "Silver Nanoparticles from Ultrasonic Spray Pyrolysis of Aqueous Silver Nitrate" (PDF). Aerosol Science and Technology. 39 (10): 1010–1014. Bibcode:2005AerST..39.1010P. doi:10.1080/02786820500380255. S2CID 6908181. Archived (PDF) from the original on 2014-04-08.
- ^ Song, Y. L.; Tsai, S. C.; Chen, C. Y.; Tseng, T. K.; Tsai, C. S.; Chen, J. W.; Yao, Y. D. (2004). "Ultrasonic Spray Pyrolysis for Synthesis of Spherical Zirconia Particles" (PDF). Journal of the American Ceramic Society. 87 (10): 1864–1871. doi:10.1111/j.1151-2916.2004.tb06332.x. Archived (PDF) from the original on 2014-04-08.
- ^ Hamedani, Hoda Amani (2008) Investigation of Deposition Parameters in Ultrasonic Spray Pyrolysis for Fabrication of Solid Oxide Fuel Cell Cathode Archived 2016-03-05 at the Wayback Machine, Georgia Institute of Technology
- ^ Barbero-López, Aitor; Chibily, Soumaya; Tomppo, Laura; Salami, Ayobami; Ancin-Murguzur, Francisco Javier; Venäläinen, Martti; Lappalainen, Reijo; Haapala, Antti (2019-03-01). "Pyrolysis distillates from tree bark and fibre hemp inhibit the growth of wood-decaying fungi". Industrial Crops and Products. 129: 604–610. doi:10.1016/j.indcrop.2018.12.049. ISSN 0926-6690.
- ^ Barbero-López, Aitor; Akkanen, Jarkko; Lappalainen, Reijo; Peräniemi, Sirpa; Haapala, Antti (2021-01-20). "Bio-based wood preservatives: Their efficiency, leaching and ecotoxicity compared to a commercial wood preservative". Science of the Total Environment. 753: 142013. Bibcode:2021ScTEn.75342013B. doi:10.1016/j.scitotenv.2020.142013. ISSN 0048-9697. PMID 32890867.
- ^ Zhou, Hui; Wu, Chunfei; Onwudili, Jude A.; Meng, Aihong; Zhang, Yanguo; Williams, Paul T. (February 2015). "Polycyclic aromatic hydrocarbons (PAH) formation from the pyrolysis of different municipal solid waste fractions" (PDF). Waste Management. 36: 136–146. Bibcode:2015WaMan..36..136Z. doi:10.1016/j.wasman.2014.09.014. PMID 25312776.
- ^ Zhou, Hui; Wu, Chunfei; Onwudili, Jude A.; Meng, Aihong; Zhang, Yanguo; Williams, Paul T. (2014-10-16). "Polycyclic Aromatic Hydrocarbon Formation from the Pyrolysis/Gasification of Lignin at Different Reaction Conditions". Energy & Fuels. 28 (10): 6371–6379. doi:10.1021/ef5013769. ISSN 0887-0624.
- ^ Zhou, Hui; Wu, Chunfei; Onwudili, Jude A.; Meng, Aihong; Zhang, Yanguo; Williams, Paul T. (April 2016). "Influence of process conditions on the formation of 2–4 ring polycyclic aromatic hydrocarbons from the pyrolysis of polyvinyl chloride" (PDF). Fuel Processing Technology. 144: 299–304. Bibcode:2016FuPrT.144..299Z. doi:10.1016/j.fuproc.2016.01.013. S2CID 55051115.
- ^ Zhou, Hui; Meng, AiHong; Long, YanQiu; Li, QingHai; Zhang, YanGuo (July 2014). "Interactions of municipal solid waste components during pyrolysis: A TG-FTIR study". Journal of Analytical and Applied Pyrolysis. 108: 19–25. Bibcode:2014JAAP..108...19Z. doi:10.1016/j.jaap.2014.05.024.
- ^ Long, Yanqiu; Zhou, Hui; Meng, Aihong; Li, Qinghai; Zhang, Yanguo (September 2016). "Interactions among biomass components during co-pyrolysis in (macro)thermogravimetric analyzers". Korean Journal of Chemical Engineering. 33 (9): 2638–2643. doi:10.1007/s11814-016-0102-x. ISSN 0256-1115. S2CID 59127489.
- ^ Goodacre, R.; Kell, D. B. (1996). "Pyrolysis mass spectrometry and its applications in biotechnology". Curr. Opin. Biotechnol. 7 (1): 20–28. doi:10.1016/S0958-1669(96)80090-5. PMID 8791308.
- ^ Peacock, P. M.; McEwen, C. N. (2006). "Mass Spectrometry of Synthetic Polymers. Anal. Chem". Analytical Chemistry. 78 (12): 3957–3964. doi:10.1021/ac0606249. PMID 16771534.
- ^ Wang, Zhengxin; Peng, Xinggan; Xia, Ao; Shah, Akeel A.; Huang, Yun; Zhu, Xianqing; Zhu, Xun; Liao, Qiang (January 2022). "The role of machine learning to boost the bioenergy and biofuels conversion". Bioresource Technology. 343: 126099. Bibcode:2022BiTec.34326099W. doi:10.1016/j.biortech.2021.126099. PMID 34626766. S2CID 238532544.
- ^ Akinpelu, David Akorede; Adekoya, Oluwaseun A.; Oladoye, Peter Olusakin; Ogbaga, Chukwuma C.; Okolie, Jude A. (September 2023). "Machine learning applications in biomass pyrolysis: From biorefinery to end-of-life product management". Digital Chemical Engineering. 8: 100103. doi:10.1016/j.dche.2023.100103. S2CID 258755762.
External links
[edit]| History | |
|---|---|
| Science | |
| Components | |
| Individual fires | |
| Crime | |
| People | |
| Culture | |
| Organizations | |
| Other | |


