| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Chloro perchlorate[1]
| |||
| Systematic IUPAC name
Chloro perchlorate[1] | |||
| Other names
Chlorine(I,VII) oxide
Dichlorine tetroxide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| Cl2O4 | |||
| Molar mass | 134.90 g·mol−1 | ||
| Appearance | Pale green liquid | ||
| Density | 1.81 g·cm−3 | ||
| Melting point | −117 °C (−179 °F; 156 K) | ||
| Boiling point | 20 °C (68 °F; 293 K) (decomposes) | ||
| Reacts | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
oxidizer | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
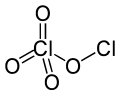
Chlorine perchlorate is a chemical compound with the formula Cl2O4. This chlorine oxide is an asymmetric oxide, with one chlorine atom in +1 oxidation state and the other +7, with proper formula ClOClO3. It is produced by the photodimerization of chlorine dioxide (ClO2) at room temperature by 436 nm ultraviolet light:[2][3][4]
- 2ClO2 → ClOClO3
Chlorine perchlorate can also be made by the following reaction at −45 °C.
- CsClO4 + ClOSO2F → Cs(SO3)F + ClOClO3