 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Unaffected by food[1] |
| Metabolism | Hepatic glucuronidation |
| Elimination half-life | ~20 hours |
| Excretion | Renal (≤30%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.243.911 |
| Chemical and physical data | |
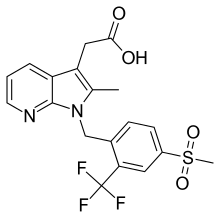
| Formula | C19H17F3N2O4S |
| Molar mass | 426.41 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fevipiprant (INN; code name QAW039) is a drug of the piprant class that was being developed by Novartis. It is a selective, orally available antagonist of the prostaglandin D2 receptor 2 (DP2 or CRTh2).[1][2][3]
By 2016 it had advanced to phase III[4] clinical trials for the treatment of asthma.[5] However, in 2019 Novartis announced that it was removing fevipiprant from its development program, given that the medicine has failed in two clinical trials in patients with moderate-to-severe asthma. The firm said that it had hoped fevipiprant would be a billion-dollar-selling asthma drug.[6]