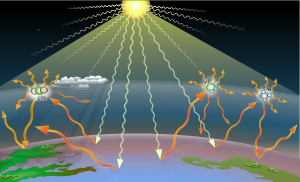

Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. What distinguishes them from other gases is that they absorb the wavelengths of radiation that a planet emits, resulting in the greenhouse effect.[1] The Earth is warmed by sunlight, causing its surface to radiate heat, which is then mostly absorbed by greenhouse gases. Without greenhouse gases in the atmosphere, the average temperature of Earth's surface would be about −18 °C (0 °F),[2] rather than the present average of 15 °C (59 °F).[3][4]
The five most abundant greenhouse gases in Earth's atmosphere, listed in decreasing order of average global mole fraction, are:[5][6] water vapor, carbon dioxide, methane, nitrous oxide, ozone. Other greenhouse gases of concern include chlorofluorocarbons (CFCs and HCFCs), hydrofluorocarbons (HFCs), perfluorocarbons, SF
6, and NF
3. Water vapor causes about half of the greenhouse effect, acting in response to other gases as a climate change feedback.[7]
Human activities since the beginning of the Industrial Revolution (around 1750) have increased carbon dioxide by over 50%,[8] and methane levels by 150%.[9] Carbon dioxide emissions are causing about three-quarters of global warming, while methane emissions cause most of the rest.[10] The vast majority of carbon dioxide emissions by humans come from the burning of fossil fuels,[11] with remaining contributions from agriculture and industry.[12]: 687 Methane emissions originate from agriculture, fossil fuel production, waste, and other sources.[13] The carbon cycle takes thousands of years to fully absorb CO2 from the atmosphere,[14] while methane lasts in the atmosphere for an average of only 12 years.[15]
Natural flows of carbon happen between the atmosphere, terrestrial ecosystems, the ocean, and sediments. These flows have been fairly balanced over the past 1 million years,[16] although greenhouse gas levels have varied widely in the more distant past. Carbon dioxide levels are now higher than they have been for 3 million years.[17] If current emission rates continue then global warming will surpass 2.0 °C (3.6 °F) sometime between 2040 and 2070. This is a level which the Intergovernmental Panel on Climate Change (IPCC) says is "dangerous".[18]
Properties and mechanisms
[edit]
Greenhouse gases are infrared active, meaning that they absorb and emit infrared radiation in the same long wavelength range as what is emitted by the Earth's surface, clouds and atmosphere.[19]: 2233
99% of the Earth's dry atmosphere (excluding water vapor) is made up of nitrogen (N
2) (78%) and oxygen (O
2) (21%). Because their molecules contain two atoms of the same element, they have no asymmetry in the distribution of their electrical charges,[20] and so are almost totally unaffected by infrared thermal radiation,[21] with only an extremely minor effect from collision-induced absorption.[22][23][24] A further 0.9% of the atmosphere is made up by argon (Ar), which is monatomic, and so completely transparent to thermal radiation. On the other hand, carbon dioxide (0.04%), methane, nitrous oxide and even less abundant trace gases account for less than 0.1% of Earth's atmosphere, but because their molecules contain atoms of different elements, there is an asymmetry in electric charge distribution which allows molecular vibrations to interact with electromagnetic radiation. This makes them infrared active, and so their presence causes greenhouse effect.[20]
Radiative forcing
[edit]
Earth absorbs some of the radiant energy received from the sun, reflects some of it as light and reflects or radiates the rest back to space as heat. A planet's surface temperature depends on this balance between incoming and outgoing energy. When Earth's energy balance is shifted, its surface becomes warmer or cooler, leading to a variety of changes in global climate.[25] Radiative forcing is a metric calculated in watts per square meter, which characterizes the impact of an external change in a factor that influences climate. It is calculated as the difference in top-of-atmosphere (TOA) energy balance immediately caused by such an external change. A positive forcing, such as from increased concentrations of greenhouse gases, means more energy arriving than leaving at the top-of-atmosphere, which causes additional warming, while negative forcing, like from sulfates forming in the atmosphere from sulfur dioxide, leads to cooling.[19]: 2245 [26]
Within the lower atmosphere, greenhouse gases exchange thermal radiation with the surface and limit radiative heat flow away from it, which reduces the overall rate of upward radiative heat transfer.[27]: 139 [28] The increased concentration of greenhouse gases is also cooling the upper atmosphere, as it is much thinner than the lower layers, and any heat re-emitted from greenhouse gases is more likely to travel further to space than to interact with the fewer gas molecules in the upper layers. The upper atmosphere is also shrinking as the result.[29]
Contributions of specific gases to the greenhouse effect
[edit]Anthropogenic changes to the natural greenhouse effect are sometimes referred to as the enhanced greenhouse effect.[19]: 2223
This table shows the most important contributions to the overall greenhouse effect, without which the average temperature of Earth's surface would be about −18 °C (0 °F),[2] instead of around 15 °C (59 °F).[3] This table also specifies tropospheric ozone, because this gas has a cooling effect in the stratosphere, but a warming influence comparable to nitrous oxide and CFCs in the troposphere.[30]
| K&T (1997)[31] | Schmidt (2010)[32] | |||
|---|---|---|---|---|
| Contributor | Clear Sky | With Clouds | Clear Sky | With Clouds |
| Water vapor | 60 | 41 | 67 | 50 |
| Clouds | 31 | 25 | ||
| CO2 | 26 | 18 | 24 | 19 |
| Tropospheric ozone (O3) | 8 | |||
| N2O + CH4 | 6 | |||
| Other | 9 | 9 | 7 | |
|
K&T (1997) used 353 ppm CO2 and calculated 125 W/m2 total clear-sky greenhouse effect; relied on single atmospheric profile and cloud model. "With Clouds" percentages are from Schmidt (2010) interpretation of K&T (1997). | ||||
Special role of water vapor
[edit]
Water vapor is the most important greenhouse gas overall, being responsible for 41–67% of the greenhouse effect,[31][32] but its global concentrations are not directly affected by human activity. While local water vapor concentrations can be affected by developments such as irrigation, it has little impact on the global scale due to its short residence time of about nine days.[34] Indirectly, an increase in global temperatures cause will also increase water vapor concentrations and thus their warming effect, in a process known as water vapor feedback. It occurs because Clausius–Clapeyron relation establishes that more water vapor will be present per unit volume at elevated temperatures.[35] Thus, local atmospheric concentration of water vapor varies from less than 0.01% in extremely cold regions and up to 3% by mass in saturated air at about 32 °C.[36]
Global warming potential (GWP) and CO2 equivalents
[edit]
Global warming potential (GWP) is an index to measure how much infrared thermal radiation a greenhouse gas would absorb over a given time frame after it has been added to the atmosphere (or emitted to the atmosphere). The GWP makes different greenhouse gases comparable with regard to their "effectiveness in causing radiative forcing".[37]: 2232 It is expressed as a multiple of the radiation that would be absorbed by the same mass of added carbon dioxide (CO2), which is taken as a reference gas. Therefore, the GWP has a value of 1 for CO2. For other gases it depends on how strongly the gas absorbs infrared thermal radiation, how quickly the gas leaves the atmosphere, and the time frame being considered.
For example, methane has a GWP over 20 years (GWP-20) of 81.2[38] meaning that, for example, a leak of a tonne of methane is equivalent to emitting 81.2 tonnes of carbon dioxide measured over 20 years. As methane has a much shorter atmospheric lifetime than carbon dioxide, its GWP is much less over longer time periods, with a GWP-100 of 27.9 and a GWP-500 of 7.95.[38]: 7SM-24
The carbon dioxide equivalent (CO2e or CO2eq or CO2-e or CO2-eq) can be calculated from the GWP. For any gas, it is the mass of CO2 that would warm the earth as much as the mass of that gas. Thus it provides a common scale for measuring the climate effects of different gases. It is calculated as GWP times mass of the other gas.List of all greenhouse gases
[edit]
The contribution of each gas to the enhanced greenhouse effect is determined by the characteristics of that gas, its abundance, and any indirect effects it may cause. For example, the direct radiative effect of a mass of methane is about 84 times stronger than the same mass of carbon dioxide over a 20-year time frame.[42] Since the 1980s, greenhouse gas forcing contributions (relative to year 1750) are also estimated with high accuracy using IPCC-recommended expressions derived from radiative transfer models.[43]
The concentration of a greenhouse gas is typically measured in parts per million (ppm) or parts per billion (ppb) by volume. A CO2 concentration of 420 ppm means that 420 out of every million air molecules is a CO2 molecule. The first 30 ppm increase in CO2 concentrations took place in about 200 years, from the start of the Industrial Revolution to 1958; however the next 90 ppm increase took place within 56 years, from 1958 to 2014.[8][44][45] Similarly, the average annual increase in the 1960s was only 37% of what it was in 2000 through 2007.[46]
Many observations are available online in a variety of Atmospheric Chemistry Observational Databases. The table below shows the most influential long-lived, well-mixed greenhouse gases, along with their tropospheric concentrations and direct radiative forcings, as identified by the Intergovernmental Panel on Climate Change (IPCC).[47] Abundances of these trace gases are regularly measured by atmospheric scientists from samples collected throughout the world.[48][49][50] It excludes water vapor because changes in its concentrations are calculated as a climate change feedback indirectly caused by changes in other greenhouse gases, as well as ozone, whose concentrations are only modified indirectly by various refrigerants that cause ozone depletion. Some short-lived gases (e.g. carbon monoxide, NOx) and aerosols (e.g. mineral dust or black carbon) are also excluded because of limited role and strong variation, alongwith minor refrigerants and other halogenated gases, which have been mass-produced in smaller quantities than those in the table.[47]: 731–738 and Annex III of the 2021 IPCC WG1 Report[51]: 4–9
| Species | Lifetime
(years) [47]: 731 |
100-yr | Mole Fraction [ppt – except as noted]a + Radiative forcing [W m−2] [B] | Concentrations
up to year 2022 | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline
Year 1750 |
TAR[54]
Year 1998 |
AR4[55]
Year 2005 |
AR5[47]: 678
Year 2011 |
AR6[51]: 4–9
Year 2019 | ||||
| CO2 [ppm] | [A] | 1 | 278 | 365 (1.46) | 379 (1.66) | 391 (1.82) | 410 (2.16) | 
|
| CH4 [ppb] | 12.4 | 28 | 700 | 1,745 (0.48) | 1,774 (0.48) | 1,801 (0.48) | 1866 (0.54) | 
|
| N2O [ppb] | 121 | 265 | 270 | 314 (0.15) | 319 (0.16) | 324 (0.17) | 332 (0.21) | 
|
| CFC-11 | 45 | 4,660 | 0 | 268 (0.07) | 251 (0.063) | 238 (0.062) | 226 (0.066) | 
|
| CFC-12 | 100 | 10,200 | 0 | 533 (0.17) | 538 (0.17) | 528 (0.17) | 503 (0.18) | 
|
| CFC-13 | 640 | 13,900 | 0 | 4 (0.001) | – | 2.7 (0.0007) | 3.28 (0.0009) | cfc13 |
| CFC-113 | 85 | 6,490 | 0 | 84 (0.03) | 79 (0.024) | 74 (0.022) | 70 (0.021) | 
|
| CFC-114 | 190 | 7,710 | 0 | 15 (0.005) | – | – | 16 (0.005) | cfc114 |
| CFC-115 | 1,020 | 5,860 | 0 | 7 (0.001) | – | 8.37 (0.0017) | 8.67 (0.0021) | cfc115 |
| HCFC-22 | 11.9 | 5,280 | 0 | 132 (0.03) | 169 (0.033) | 213 (0.0447) | 247 (0.0528) | 
|
| HCFC-141b | 9.2 | 2,550 | 0 | 10 (0.001) | 18 (0.0025) | 21.4 (0.0034) | 24.4 (0.0039) | 
|
| HCFC-142b | 17.2 | 5,020 | 0 | 11 (0.002) | 15 (0.0031) | 21.2 (0.0040) | 22.3 (0.0043) | 
|
| CH3CCl3 | 5 | 160 | 0 | 69 (0.004) | 19 (0.0011) | 6.32 (0.0004) | 1.6 (0.0001) | 
|
| CCl4 | 26 | 1,730 | 0 | 102 (0.01) | 93 (0.012) | 85.8 (0.0146) | 78 (0.0129) | 
|
| HFC-23 | 222 | 12,400 | 0 | 14 (0.002) | 18 (0.0033) | 24 (0.0043) | 32.4 (0.0062) | 
|
| HFC-32 | 5.2 | 677 | 0 | – | – | 4.92 (0.0005) | 20 (0.0022) | 
|
| HFC-125 | 28.2 | 3,170 | 0 | – | 3.7 (0.0009) | 9.58 (0.0022) | 29.4 (0.0069) | 
|
| HFC-134a | 13.4 | 1,300 | 0 | 7.5 (0.001) | 35 (0.0055) | 62.7 (0.0100) | 107.6 (0.018) | 
|
| HFC-143a | 47.1 | 4,800 | 0 | – | – | 12.0 (0.0019) | 24 (0.0040) | 
|
| HFC-152a | 1.5 | 138 | 0 | 0.5 (0.0000) | 3.9 (0.0004) | 6.4 (0.0006) | 7.1 (0.0007) | 
|
| CF4 (PFC-14) | 50,000 | 6,630 | 40 | 80 (0.003) | 74 (0.0034) | 79 (0.0040) | 85.5 (0.0051) | 
|
| C2F6 (PFC-116) | 10,000 | 11,100 | 0 | 3 (0.001) | 2.9 (0.0008) | 4.16 (0.0010) | 4.85 (0.0013) | 
|
| SF6 | 3,200 | 23,500 | 0 | 4.2 (0.002) | 5.6 (0.0029) | 7.28 (0.0041) | 9.95 (0.0056) | 
|
| SO2F2 | 36 | 4,090 | 0 | – | – | 1.71 (0.0003) | 2.5 (0.0005) | 
|
| NF3 | 500 | 16,100 | 0 | – | – | 0.9 (0.0002) | 2.05 (0.0004) | 
|
a Mole fractions: μmol/mol = ppm = parts per million (106); nmol/mol = ppb = parts per billion (109); pmol/mol = ppt = parts per trillion (1012).
A The IPCC states that "no single atmospheric lifetime can be given" for CO2.[47]: 731 This is mostly due to the rapid growth and cumulative magnitude of the disturbances to Earth's carbon cycle by the geologic extraction and burning of fossil carbon.[56] As of year 2014, fossil CO2 emitted as a theoretical 10 to 100 GtC pulse on top of the existing atmospheric concentration was expected to be 50% removed by land vegetation and ocean sinks in less than about a century, as based on the projections of coupled models referenced in the AR5 assessment.[57] A substantial fraction (20–35%) was also projected to remain in the atmosphere for centuries to millennia, where fractional persistence increases with pulse size.[58][59]
B Values are relative to year 1750. AR6 reports the effective radiative forcing which includes effects of rapid adjustments in the atmosphere and at the surface.[60]
Factors affecting concentrations
[edit]Atmospheric concentrations are determined by the balance between sources (emissions of the gas from human activities and natural systems) and sinks (the removal of the gas from the atmosphere by conversion to a different chemical compound or absorption by bodies of water).[61]: 512
Airborne fraction
[edit]
The proportion of an emission remaining in the atmosphere after a specified time is the "airborne fraction" (AF). The annual airborne fraction is the ratio of the atmospheric increase in a given year to that year's total emissions. The annual airborne fraction for CO2 had been stable at 0.45 for the past six decades even as the emissions have been increasing. This means that the other 0.55 of emitted CO2 is absorbed by the land and atmosphere carbon sinks within the first year of an emission.[56] In the high-emission scenarios, the effectiveness of carbon sinks will be lower, increasing the atmospheric fraction of CO2 even though the raw amount of emissions absorbed will be higher than in the present.[62]: 746
Atmospheric lifetime
[edit]
Major greenhouse gases are well mixed and take many years to leave the atmosphere.[64]
The atmospheric lifetime of a greenhouse gas refers to the time required to restore equilibrium following a sudden increase or decrease in its concentration in the atmosphere. Individual atoms or molecules may be lost or deposited to sinks such as the soil, the oceans and other waters, or vegetation and other biological systems, reducing the excess to background concentrations. The average time taken to achieve this is the mean lifetime. This can be represented through the following formula, where the lifetime of an atmospheric species X in a one-box model is the average time that a molecule of X remains in the box.[65]
can also be defined as the ratio of the mass (in kg) of X in the box to its removal rate, which is the sum of the flow of X out of the box (), chemical loss of X (), and deposition of X () (all in kg/s):
- .[65]
If input of this gas into the box ceased, then after time , its concentration would decrease by about 63%.
Changes to any of these variables can alter the atmospheric lifetime of a greenhouse gas. For instance, methane's atmospheric lifetime is estimated to have been lower in the 19th century than now, but to have been higher in the second half of the 20th century than after 2000.[63] Carbon dioxide has an even more variable lifetime, which cannot be specified down to a single number.[66][42][19]: 2237 Scientists instead say that while the first 10% of carbon dioxide's airborne fraction (not counting the ~50% absorbed by land and ocean sinks within the emission's first year) is removed "quickly", the vast majority of the airborne fraction – 80% – lasts for "centuries to millennia". The remaining 10% stays for tens of thousands of years. In some models, this longest-lasting fraction is as large as 30%.[67][68]
During geologic time scales
[edit]

Estimates in 2023 found that the current carbon dioxide concentration in the atmosphere may be the highest it has been in the last 14 million years.[69] However the IPCC Sixth Assessment Report estimated similar levels 3 to 3.3 million years ago in the mid-Pliocene warm period. This period can be a proxy for likely climate outcomes with current levels of CO2.[70]: Figure 2.34
Carbon dioxide is believed to have played an important effect in regulating Earth's temperature throughout its 4.54 billion year history. Early in the Earth's life, scientists have found evidence of liquid water indicating a warm world even though the Sun's output is believed to have only been 70% of what it is today. Higher carbon dioxide concentrations in the early Earth's atmosphere might help explain this faint young sun paradox. When Earth first formed, Earth's atmosphere may have contained more greenhouse gases and CO2 concentrations may have been higher, with estimated partial pressure as large as 1,000 kPa (10 bar), because there was no bacterial photosynthesis to reduce the gas to carbon compounds and oxygen. Methane, a very active greenhouse gas, may have been more prevalent as well.[71][72]Monitoring
[edit]
Greenhouse gas monitoring involves the direct measurement of atmospheric concentrations and direct and indirect measurement of greenhouse gas emissions. Indirect methods calculate emissions of greenhouse gases based on related metrics such as fossil fuel extraction.[56]
There are several different methods of measuring carbon dioxide concentrations in the atmosphere, including infrared analyzing and manometry.[74] Methane and nitrous oxide are measured by other instruments, such as the range-resolved infrared differential absorption lidar (DIAL).[75] Greenhouse gases are measured from space such as by the Orbiting Carbon Observatory and through networks of ground stations such as the Integrated Carbon Observation System.[56]
The Annual Greenhouse Gas Index (AGGI) is defined by atmospheric scientists at NOAA as the ratio of total direct radiative forcing due to long-lived and well-mixed greenhouse gases for any year for which adequate global measurements exist, to that present in year 1990.[41][76] These radiative forcing levels are relative to those present in year 1750 (i.e. prior to the start of the industrial era). 1990 is chosen because it is the baseline year for the Kyoto Protocol, and is the publication year of the first IPCC Scientific Assessment of Climate Change. As such, NOAA states that the AGGI "measures the commitment that (global) society has already made to living in a changing climate. It is based on the highest quality atmospheric observations from sites around the world. Its uncertainty is very low."[77]
Data networks
[edit]Types of sources
[edit]Natural sources
[edit]The natural flows of carbon between the atmosphere, ocean, terrestrial ecosystems, and sediments are fairly balanced; so carbon levels would be roughly stable without human influence.[82][83] Carbon dioxide is removed from the atmosphere primarily through photosynthesis and enters the terrestrial and oceanic biospheres. Carbon dioxide also dissolves directly from the atmosphere into bodies of water (ocean, lakes, etc.), as well as dissolving in precipitation as raindrops fall through the atmosphere. When dissolved in water, carbon dioxide reacts with water molecules and forms carbonic acid, which contributes to ocean acidity. It can then be absorbed by rocks through weathering. It also can acidify other surfaces it touches or be washed into the ocean.[84]

Human-made sources
[edit]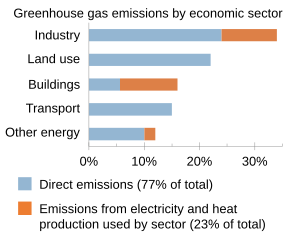
The vast majority of carbon dioxide emissions by humans come from the burning of fossil fuels. Additional contributions come from cement manufacturing, fertilizer production, and changes in land use like deforestation.[12]: 687 [11][88] Methane emissions originate from agriculture, fossil fuel production, waste, and other sources.[13]
If current emission rates continue then temperature rises will surpass 2.0 °C (3.6 °F) sometime between 2040 and 2070, which is the level the United Nations' Intergovernmental Panel on Climate Change (IPCC) says is "dangerous".[18]
Most greenhouse gases have both natural and human-caused sources. An exception are purely human-produced synthetic halocarbons which have no natural sources. During the pre-industrial Holocene, concentrations of existing gases were roughly constant, because the large natural sources and sinks roughly balanced. In the industrial era, human activities have added greenhouse gases to the atmosphere, mainly through the burning of fossil fuels and clearing of forests.[89][4]: 115
The major anthropogenic (human origin) sources of greenhouse gases are carbon dioxide (CO2), nitrous oxide (N
2O), methane, three groups of fluorinated gases (sulfur hexafluoride (SF
6), hydrofluorocarbons (HFCs) and perfluorocarbons (PFCs, sulphur hexafluoride (SF6), and nitrogen trifluoride (NF3)).[90] Though the greenhouse effect is heavily driven by water vapor,[91] human emissions of water vapor are not a significant contributor to warming.
Needed emissions cuts
[edit]
The annual "Emissions Gap Report" by UNEP stated in 2022 that it was necessary to almost halve emissions. "To get on track for limiting global warming to 1.5°C, global annual GHG emissions must be reduced by 45 per cent compared with emissions projections under policies currently in place in just eight years, and they must continue to decline rapidly after 2030, to avoid exhausting the limited remaining atmospheric carbon budget."[97]: xvi The report commented that the world should focus on broad-based economy-wide transformations and not incremental change.[97]: xvi
In 2022, the Intergovernmental Panel on Climate Change (IPCC) released its Sixth Assessment Report on climate change. It warned that greenhouse gas emissions must peak before 2025 at the latest and decline 43% by 2030 to have a good chance of limiting global warming to 1.5 °C (2.7 °F).[98][99] Or in the words of Secretary-General of the United Nations António Guterres: "Main emitters must drastically cut emissions starting this year".[100]Removal from the atmosphere through negative emissions
[edit]A number of technologies remove greenhouse gases emissions from the atmosphere. Most widely analyzed are those that remove carbon dioxide from the atmosphere, either to geologic formations such as bio-energy with carbon capture and storage and carbon dioxide air capture,[101] or to the soil as in the case with biochar.[101] Many long-term climate scenario models require large-scale human-made negative emissions to avoid serious climate change.[102]
Negative emissions approaches are also being studied for atmospheric methane, called atmospheric methane removal.[103]
History of discovery
[edit]
In the late 19th century, scientists experimentally discovered that N
2 and O
2 do not absorb infrared radiation (called, at that time, "dark radiation"), while water (both as true vapor and condensed in the form of microscopic droplets suspended in clouds) and CO2 and other poly-atomic gaseous molecules do absorb infrared radiation.[105][106] In the early 20th century, researchers realized that greenhouse gases in the atmosphere made Earth's overall temperature higher than it would be without them. The term greenhouse was first applied to this phenomenon by Nils Gustaf Ekholm in 1901.[107][108]
During the late 20th century, a scientific consensus evolved that increasing concentrations of greenhouse gases in the atmosphere cause a substantial rise in global temperatures and changes to other parts of the climate system,[109] with consequences for the environment and for human health.
Other planets
[edit]Greenhouse gases exist in many atmospheres, creating greenhouse effects on Mars, Titan and particularly in the thick atmosphere of Venus.[110] While Venus has been described as the ultimate end state of runaway greenhouse effect, such a process would have virtually no chance of occurring from any increases in greenhouse gas concentrations caused by humans,[111] as the Sun's brightness is too low and it would likely need to increase by some tens of percents, which will take a few billion years.[112]
See also
[edit]References
[edit]- ^ Matthews, J.B.R.; Möller, V.; van Diemenn, R.; Fuglesvedt, J.R.; et al. (9 August 2021). "Annex VII: Glossary". In Masson-Delmotte, Valérie; Zhai, Panmao; Pirani, Anna; Connors, Sarah L.; Péan, Clotilde; et al. (eds.). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (PDF). IPCC / Cambridge University Press. pp. 2215–2256. doi:10.1017/9781009157896.022. ISBN 9781009157896.
- ^ a b Qiancheng Ma (March 1998). "Science Briefs: Greenhouse Gases: Refining the Role of Carbon Dioxide". NASA GISS. Archived from the original on 12 January 2005. Retrieved 26 April 2016.
- ^ a b Karl TR, Trenberth KE (2003). "Modern global climate change". Science. 302 (5651): 1719–23. Bibcode:2003Sci...302.1719K. doi:10.1126/science.1090228. PMID 14657489. S2CID 45484084. Archived from the original on 22 April 2021. Retrieved 26 July 2019 – via Zenodo.
- ^ a b Le Treut, H., R. Somerville, U. Cubasch, Y. Ding, C. Mauritzen, A. Mokssit, T. Peterson and M. Prather, 2007: "Chapter 1: Historical Overview of Climate Change". In: "Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change". [Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor and H.L. Miller (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- ^ "Atmospheric Concentration of Greenhouse Gases" (PDF). U.S. Environmental Protection Agency. 1 August 2016. Archived (PDF) from the original on 19 October 2021. Retrieved 6 September 2021.
- ^ "Inside the Earth's invisible blanket". sequestration.org. Archived from the original on 28 July 2020. Retrieved 5 March 2021.
- ^ Gavin Schmidt (1 October 2010). "Taking the Measure of the Greenhouse Effect". NASA Goddard Institute for Space Studies – Science Briefs.
- ^ a b "Carbon dioxide now more than 50% higher than pre-industrial levels". National Oceanic and Atmospheric Administration. 3 June 2022. Retrieved 30 August 2022.
- ^ "Understanding methane emissions". International Energy Agency.
The concentration of methane in the atmosphere is currently over two-and-a-half times greater than its pre-industrial levels
- ^ "Global Greenhouse Gas Emissions Data". United States Environmental Protection Agency. 12 January 2016.
- ^ a b "Global Greenhouse Gas Emissions Data". U.S. Environmental Protection Agency. 12 January 2016. Archived from the original on 5 December 2019. Retrieved 30 December 2019.
The burning of coal, natural gas, and oil for electricity and heat is the largest single source of global greenhouse gas emissions.
- ^ a b Canadell, J.G., P.M.S. Monteiro, M.H. Costa, L. Cotrim da Cunha, P.M. Cox, A.V. Eliseev, S. Henson, M. Ishii, S. Jaccard, C. Koven, A. Lohila, P.K. Patra, S. Piao, J. Rogelj, S. Syampungani, S. Zaehle, and K. Zickfeld, 2021: Chapter 5: Global Carbon and other Biogeochemical Cycles and Feedbacks. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 673–816, doi:10.1017/9781009157896.007.
- ^ a b "Global Methane Tracker 2023". International Energy Agency. 21 February 2023.
- ^ "Climate Change Indicators: Greenhouse Gases". United States Environmental Protection Agency. 16 December 2015.
Carbon dioxide's lifetime cannot be represented with a single value because the gas is not destroyed over time, but instead moves among different parts of the ocean–atmosphere–land system. Some of the excess carbon dioxide is absorbed quickly (for example, by the ocean surface), but some will remain in the atmosphere for thousands of years, due in part to the very slow process by which carbon is transferred to ocean sediments.
- ^ "Understanding methane emissions". International Energy Agency.
- ^ "Climate Change Indicators: Atmospheric Concentrations of Greenhouse Gases". EPA.gov. U.S. Environmental Protection Agency. Retrieved 20 June 2024.
- ^ Lindsey, Rebecca. "Climate Change: Atmospheric Carbon Dioxide". climate.gov. Archived from the original on 24 June 2013. Retrieved 2 March 2020.
- ^ a b "Analysis: When might the world exceed 1.5C and 2C of global warming?". Carbon Brief. 4 December 2020. Archived from the original on 6 June 2021. Retrieved 17 June 2021.
- ^ a b c d IPCC, 2021: Annex VII: Glossary [Matthews, J.B.R., V. Möller, R. van Diemen, J.S. Fuglestvedt, V. Masson-Delmotte, C. Méndez, S. Semenov, A. Reisinger (eds.)]. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 2215–2256, doi:10.1017/9781009157896.022.
- ^ a b Archer, David (2011). Global Warming: Understanding the Forecast, Chapter 4: Greenhouse Gases (PDF) (2 ed.). Wiley. ISBN 978-0470943410. Retrieved 14 June 2023.
- ^ Wei, Peng-Sheng; Hsieh, Yin-Chih; Chiu, Hsuan-Han; Yen, Da-Lun; Lee, Chieh; Tsai, Yi-Cheng; Ting, Te-Chuan (6 October 2018). "Absorption coefficient of carbon dioxide across atmospheric troposphere layer". Heliyon. 4 (10): e00785. Bibcode:2018Heliy...400785W. doi:10.1016/j.heliyon.2018.e00785. PMC 6174548. PMID 30302408.
- ^ Höpfner, M.; Milz, M.; Buehler, S.; Orphall, J.; Stiller, G. (24 May 2012). "The natural greenhouse effect of atmospheric oxygen (O2) and nitrogen (N2)". Geophysical Research Letters. 39 (L10706). Bibcode:2012GeoRL..3910706H. doi:10.1029/2012GL051409. ISSN 1944-8007. S2CID 128823108.
- ^ "Which Gases Are Greenhouse Gases?". American Chemical Society. Retrieved 31 May 2021.
- ^ Höpfner, M.; Milz, M.; Buehler, S.; Orphall, J.; Stiller, G. (24 May 2012). "The natural greenhouse effect of atmospheric oxygen (O2) and nitrogen (N2)". Geophysical Research Letters. 39 (L10706). Bibcode:2012GeoRL..3910706H. doi:10.1029/2012GL051409. ISSN 1944-8007. S2CID 128823108.
- ^ "Climate Change Indicators in the United States – Greenhouse Gases". U.S. Environmental Protection Agency (EPA). 2016. Archived from the original on 27 August 2016. Retrieved 5 September 2020..
- ^ "Climate Change Indicators in the United States – Climate Forcing". U.S. Environmental Protection Agency (EPA). 2016. Archived from the original on 27 August 2016. Retrieved 5 September 2020.[1] Archived 21 September 2020 at the Wayback Machine
- ^ Wallace, J. M.; Hobbs, P. V. (2006). Atmospheric Science (2 ed.). Academic Press. ISBN 978-0-12-732951-2.
- ^ Manabe, S.; Strickler, R. F. (1964). "Thermal Equilibrium of the Atmosphere with a Convective Adjustment". J. Atmos. Sci. 21 (4): 361–385. Bibcode:1964JAtS...21..361M. doi:10.1175/1520-0469(1964)021<0361:TEOTAW>2.0.CO;2.
- ^ Hatfield, Miles (30 June 2021). "NASA Satellites See Upper Atmosphere Cooling and Contracting Due to Climate Change". NASA.
- ^ "Atmospheric Concentration of Greenhouse Gases" (PDF). U.S. Environmental Protection Agency. 1 August 2016.
- ^ a b Kiehl, J.T.; Kevin E. Trenberth (1997). "Earth's annual global mean energy budget" (PDF). Bulletin of the American Meteorological Society. 78 (2): 197–208. Bibcode:1997BAMS...78..197K. doi:10.1175/1520-0477(1997)078<0197:EAGMEB>2.0.CO;2.
- ^ a b Schmidt, G.A.; R. Ruedy; R.L. Miller; A.A. Lacis (2010), "The attribution of the present-day total greenhouse effect" (PDF), J. Geophys. Res., vol. 115, no. D20, pp. D20106, Bibcode:2010JGRD..11520106S, doi:10.1029/2010JD014287, archived from the original (PDF) on 22 October 2011, D20106. Web page Archived 4 June 2012 at the Wayback Machine
- ^ "NASA: Climate Forcings and Global Warming". 14 January 2009. Archived from the original on 18 April 2021. Retrieved 20 April 2014.
- ^ "AGU Water Vapor in the Climate System". Eso.org. 27 April 1995. Archived from the original on 20 October 2012. Retrieved 11 September 2011.
- ^ Held, Isaac M.; Soden, Brian J. (November 2000). "Water vapor feedback and global warming". Annual Review of Energy and the Environment. 25 (1): 441–475. CiteSeerX 10.1.1.22.9397. doi:10.1146/annurev.energy.25.1.441. ISSN 1056-3466.
- ^ Evans, Kimberly Masters (2005). "The greenhouse effect and climate change". The environment: a revolution in attitudes. Detroit: Thomson Gale. ISBN 978-0787690823.
- ^ IPCC, 2021: Annex VII: Glossary [Matthews, J.B.R., V. Möller, R. van Diemen, J.S. Fuglestvedt, V. Masson-Delmotte, C. Méndez, S. Semenov, A. Reisinger (eds.)]. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 2215–2256, doi:10.1017/9781009157896.022.
- ^ a b 7.SM.6 Tables of greenhouse gas lifetimes, radiative efficiencies and metrics (PDF), IPCC, 2021, p. 7SM-24.
- ^ "The NOAA Annual Greenhouse Gas Index (AGGI)". NOAA.gov. National Oceanic and Atmospheric Administration (NOAA). Spring 2023. Archived from the original on 24 May 2023.
- ^ "Annual Greenhouse Gas Index". U.S. Global Change Research Program. Archived from the original on 21 April 2021. Retrieved 5 September 2020.
- ^ a b Butler J. and Montzka S. (2020). "The NOAA Annual Greenhouse Gas Index (AGGI)". NOAA Global Monitoring Laboratory/Earth System Research Laboratories. Archived from the original on 22 September 2013. Retrieved 5 September 2020.
- ^ a b "Appendix 8.A" (PDF). Intergovernmental Panel on Climate Change Fifth Assessment Report. p. 731. Archived (PDF) from the original on 13 October 2017. Retrieved 6 November 2017.
- ^ Butler J. and Montzka S. (2020). "The NOAA Annual Greenhouse Gas Index (AGGI)". NOAA Global Monitoring Laboratory/Earth System Research Laboratories.
- ^ Charles J. Kibert (2016). "Background". Sustainable Construction: Green Building Design and Delivery. Wiley. ISBN 978-1119055327.
- ^ "Full Mauna Loa CO2 record". Earth System Research Laboratories. 2005. Archived from the original on 28 April 2017. Retrieved 6 May 2017.
- ^ Tans, Pieter (3 May 2008). "Annual CO2 mole fraction increase (ppm) for 1959–2007". National Oceanic and Atmospheric Administration Earth System Research Laboratories, Global Monitoring Division. "additional details". Archived from the original on 25 December 2018. Retrieved 15 May 2008.; see also Masarie, K.A.; Tans, P.P. (1995). "Extension and integration of atmospheric carbon dioxide data into a globally consistent measurement record". J. Geophys. Res. 100 (D6): 11593–610. Bibcode:1995JGR...10011593M. doi:10.1029/95JD00859. Archived from the original on 8 March 2021. Retrieved 26 July 2019.
- ^ a b c d e f "Chapter 8". AR5 Climate Change 2013: The Physical Science Basis.
- ^ "Global Monitoring Laboratory". NOAA Earth System Research Laboratories. Retrieved 11 December 2020.
- ^ "World Data Centre for Greenhouse Gases". World Meteorological Organization Global Atmosphere Watch Programme and Japan Meteorological Agency. Retrieved 11 December 2020.
- ^ "Advanced Global Atmospheric Gas Experiment". Massachusetts Institute of Technology. Retrieved 11 December 2020.
- ^ a b Dentener F. J.; B. Hall; C. Smith, eds. (9 August 2021), "Annex III: Tables of historical and projected well-mixed greenhouse gas mixing ratios and effective radiative forcing of all climate forcers" (PDF), Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press
- ^ "Long-term global trends of atmospheric trace gases". NOAA Earth System Research Laboratories. Retrieved 11 February 2021.
- ^ "AGAGE Data and Figures". Massachusetts Institute of Technology. Retrieved 11 February 2021.
- ^ "Chapter 6". TAR Climate Change 2001: The Scientific Basis. p. 358.
- ^ "Chapter 2". AR4 Climate Change 2007: The Physical Science Basis. p. 141.
- ^ a b c d Friedlingstein, Pierre; O'Sullivan, Michael; Jones, Matthew W.; Andrew, Robbie M.; Hauck, Judith; Olsen, Are; Peters, Glen P.; Peters, Wouter; Pongratz, Julia; Sitch, Stephen; Le Quéré, Corinne; Canadell, Josep G.; Ciais, Philippe; Jackson, Robert B.; Alin, Simone (2020). "Global Carbon Budget 2020". Earth System Science Data. 12 (4): 3269–3340. Bibcode:2020ESSD...12.3269F. doi:10.5194/essd-12-3269-2020. hdl:20.500.11850/458765. ISSN 1866-3516.
- ^ "Figure 8.SM.4" (PDF). Intergovernmental Panel on Climate Change Fifth Assessment Report – Supplemental Material. p. 8SM-16.
- ^ Archer, David (2009). "Atmospheric lifetime of fossil fuel carbon dioxide". Annual Review of Earth and Planetary Sciences. 37 (1): 117–34. Bibcode:2009AREPS..37..117A. doi:10.1146/annurev.earth.031208.100206. hdl:2268/12933.
- ^ Joos, F.; Roth, R.; Fuglestvedt, J.D.; et al. (2013). "Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis". Atmospheric Chemistry and Physics. 13 (5): 2793–2825. doi:10.5194/acpd-12-19799-2012. hdl:20.500.11850/58316.
- ^ Hansen, J.; Sato, M.; Ruedy, R.; et al. (2005). "Efficacy of Climate Forcings". Journal of Geophysical Research: Atmospheres. 119 (D18104). Bibcode:2005JGRD..11018104H. doi:10.1029/2005JD005776.
- ^ Denman, K.L., G. Brasseur, A. Chidthaisong, P. Ciais, P.M. Cox, R.E. Dickinson, D. Hauglustaine, C. Heinze, E. Holland, D. Jacob, U. Lohmann, S Ramachandran, P.L. da Silva Dias, S.C. Wofsy and X. Zhang, 2007: Chapter 7: Couplings Between Changes in the Climate System and Biogeochemistry. In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M.Tignor and H.L. Miller (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- ^ Canadell, J. G.; Monteiro, P. M. S.; Costa, M. H.; Cotrim da Cunha, L.; Ishii, M.; Jaccard, S.; Cox, P. M.; Eliseev, A. V.; Henson, S.; Koven, C.; Lohila, A.; Patra, P. K.; Piao, S.; Rogelj, J.; Syampungani, S.; Zaehle, S.; Zickfeld, K. (2021). "Global Carbon and Other Biogeochemical Cycles and Feedbacks" (PDF). IPCC Sixth Assessment Report: Working Group 1.
- ^ a b Arora, Vivek K.; Melton, Joe R.; Plummer, David (1 August 2018). "An assessment of natural methane fluxes simulated by the CLASS-CTEM model". Biogeosciences. 15 (15): 4683–4709. Bibcode:2018BGeo...15.4683A. doi:10.5194/bg-15-4683-2018.
- ^ Betts (2001). "6.3 Well-mixed Greenhouse Gases". Chapter 6 Radiative Forcing of Climate Change. Working Group I: The Scientific Basis IPCC Third Assessment Report – Climate Change 2001. UNEP/GRID-Arendal – Publications. Archived from the original on 29 June 2011. Retrieved 16 October 2010.
- ^ a b Jacob, Daniel (1999). Introduction to atmospheric chemistry. Princeton University Press. pp. 25–26. ISBN 978-0691001852. Archived from the original on 2 September 2011.
- ^ "How long will global warming last?". RealClimate. 15 March 2005. Archived from the original on 4 March 2021. Retrieved 12 June 2012.
- ^ "How long will global warming last?". MIT Climate Portal. 17 January 2023.
- ^ Atkinson, Kate (19 July 2023). "How long will global warming last?". Australian Associated Press.
- ^ AHMED, Issam. "Current carbon dioxide levels last seen 14 million years ago". phys.org. Retrieved 8 February 2024.
- ^ Gulev, S.K., P.W. Thorne, J. Ahn, F.J. Dentener, C.M. Domingues, S. Gerland, D. Gong, D.S. Kaufman, H.C. Nnamchi, J. Quaas, J.A. Rivera, S. Sathyendranath, S.L. Smith, B. Trewin, K. von Schuckmann, and R.S. Vose, 2021: Chapter 2: Changing State of the Climate System. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 287–422, doi:10.1017/9781009157896.004.
- ^ Walker, James C.G. (June 1985). "Carbon dioxide on the early earth" (PDF). Origins of Life and Evolution of the Biosphere. 16 (2): 117–27. Bibcode:1985OrLi...16..117W. doi:10.1007/BF01809466. hdl:2027.42/43349. PMID 11542014. S2CID 206804461. Archived (PDF) from the original on 14 September 2012. Retrieved 30 January 2010.
- ^ Pavlov, Alexander A.; Kasting, James F.; Brown, Lisa L.; Rages, Kathy A.; Freedman, Richard (May 2000). "Greenhouse warming by CH4 in the atmosphere of early Earth". Journal of Geophysical Research. 105 (E5): 11981–90. Bibcode:2000JGR...10511981P. doi:10.1029/1999JE001134. PMID 11543544.
- ^ Guevara, Marc; Enciso, Santiago; Tena, Carles; Jorba, Oriol; Dellaert, Stijn; Denier van der Gon, Hugo; Pérez García-Pando, Carlos (15 January 2024). "A global catalogue of CO2 emissions and co-emitted species from power plants, including high-resolution vertical and temporal profiles". Earth System Science Data. 16 (1): 337–373. doi:10.5194/essd-16-337-2024. hdl:2117/405068.
- ^ Harris, Daniel C. (2010). "Charles David Keeling and the Story of Atmospheric CO2 Measurements". Analytical Chemistry. 82 (19): 7865–7870. doi:10.1021/ac1001492. ISSN 0003-2700. PMID 20536268.
- ^ Innocenti, Fabrizio; Robinson, Rod; Gardiner, Tom; Finlayson, Andrew; Connor, Andy (2017). "Differential Absorption Lidar (DIAL) Measurements of Landfill Methane Emissions". Remote Sensing. 9 (9): 953. Bibcode:2017RemS....9..953I. doi:10.3390/rs9090953.
- ^ LuAnn Dahlman (14 August 2020). "Climate change: annual greenhouse gas index". NOAA Climate.gov science news & Information for a climate smart nation. Archived from the original on 16 August 2013. Retrieved 5 September 2020.
- ^ "The NOAA Annual Greenhouse Gas Index (AGGI) – An Introduction". NOAA Global Monitoring Laboratory/Earth System Research Laboratories. Archived from the original on 27 November 2020. Retrieved 5 September 2020.
- ^ "NOAA CCGG page Retrieved 2 March 2016". Archived from the original on 11 August 2011. Retrieved 14 March 2023.
- ^ WDCGG webpage Archived 6 April 2016 at the Wayback Machine Retrieved 2 March 2016
- ^ RAMCES webpage [permanent dead link] Retrieved 2 March 2016
- ^ "CDIAC CO2 page Retrieved 9 February 2016". Archived from the original on 13 August 2011. Retrieved 14 March 2023.
- ^ Prentice, I.C. (2001). "The carbon cycle and atmospheric carbon dioxide". In Houghton, J.T. (ed.). Climate change 2001: the scientific basis: contribution of Working Group I to the Third Assessment Report of the Intergouvernmental Panel on Climate Change. hdl:10067/381670151162165141.
- ^ "An Introduction to the Global Carbon Cycle" (PDF). University of New Hampshire. 2009. Archived (PDF) from the original on 8 October 2016. Retrieved 6 February 2016.
- ^ "Many Planets, One Earth // Section 4: Carbon Cycling and Earth's Climate". Many Planets, One Earth. 4. Archived from the original on 17 April 2012. Retrieved 24 June 2012.
- ^ Friedlingstein, Pierre; O'Sullivan, Michael; Jones, Matthew W.; Andrew, Robbie M.; Hauck, Judith; Olsen, Are; Peters, Glen P.; Peters, Wouter; Pongratz, Julia; Sitch, Stephen; Le Quéré, Corinne; Canadell, Josep G.; Ciais, Philippe; Jackson, Robert B.; Alin, Simone (2020). "Global Carbon Budget 2020". Earth System Science Data. 12 (4): 3269–3340. Bibcode:2020ESSD...12.3269F. doi:10.5194/essd-12-3269-2020. hdl:20.500.11850/458765. ISSN 1866-3516.
- ^ Falkowski, P.; Scholes, R. J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Högberg, P.; Linder, S.; MacKenzie, F. T.; Moore III, B.; Pedersen, T.; Rosenthal, Y.; Seitzinger, S.; Smetacek, V.; Steffen, W. (2000). "The Global Carbon Cycle: A Test of Our Knowledge of Earth as a System". Science. 290 (5490): 291–296. Bibcode:2000Sci...290..291F. doi:10.1126/science.290.5490.291. PMID 11030643.
- ^ Riebeek, Holli (16 June 2011). "The Carbon Cycle". Earth Observatory. NASA. Archived from the original on 5 March 2016. Retrieved 5 April 2018.
- ^ "AR4 SYR Synthesis Report Summary for Policymakers – 2 Causes of change". ipcc.ch. Archived from the original on 28 February 2018. Retrieved 9 October 2015.
- ^ "Chapter 3, IPCC Special Report on Emissions Scenarios, 2000" (PDF). Intergovernmental Panel on Climate Change. 2000. Archived (PDF) from the original on 20 August 2018. Retrieved 16 October 2010.
- ^ Dhakal, S., J.C. Minx, F.L. Toth, A. Abdel-Aziz, M.J. Figueroa Meza, K. Hubacek, I.G.C. Jonckheere, Yong-Gun Kim, G.F. Nemet, S. Pachauri, X.C. Tan, T. Wiedmann, 2022: Chapter 2: Emissions Trends and Drivers. In IPCC, 2022: Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [P.R. Shukla, J. Skea, R. Slade, A. Al Khourdajie, R. van Diemen, D. McCollum, M. Pathak, S. Some, P. Vyas, R. Fradera, M. Belkacemi, A. Hasija, G. Lisboa, S. Luz, J. Malley, (eds.)]. Cambridge University Press, Cambridge, UK and New York, NY, USA. doi: 10.1017/9781009157926.004
- ^ "Water Vapor". earthobservatory.nasa.gov. 30 June 2023. Retrieved 16 August 2023.
- ^ Johnston, Chris; Milman, Oliver; Vidal, John (15 October 2016). "Climate change: global deal reached to limit use of hydrofluorocarbons". The Guardian. Retrieved 21 August 2018.
- ^ "Climate change: 'Monumental' deal to cut HFCs, fastest growing greenhouse gases". BBC News. 15 October 2016. Retrieved 15 October 2016.
- ^ "Nations, Fighting Powerful Refrigerant That Warms Planet, Reach Landmark Deal". The New York Times. 15 October 2016. Retrieved 15 October 2016.
- ^ Vaara, Miska (2003), Use of ozone depleting substances in laboratories, TemaNord, p. 170, ISBN 978-9289308847, archived from the original on 6 August 2011
- ^ Montreal Protocol
- ^ a b United Nations Environment Programme (2022). Emissions Gap Report 2022: The Closing Window — Climate crisis calls for rapid transformation of societies. Nairobi.
- ^ "It's over for fossil fuels: IPCC spells out what's needed to avert climate disaster". The Guardian. 4 April 2022. Retrieved 4 April 2022.
- ^ "The evidence is clear: the time for action is now. We can halve emissions by 2030". IPCC. 4 April 2022. Retrieved 4 April 2022.
- ^ "Ambitious Action Key to Resolving Triple Planetary Crisis of Climate Disruption, Nature Loss, Pollution, Secretary-General Says in Message for International Mother Earth Day | Meetings Coverage and Press Releases". www.un.org. Retrieved 10 June 2022.
- ^ a b "Geoengineering the climate: science, governance and uncertainty". The Royal Society. 2009. Archived from the original on 7 September 2009. Retrieved 12 September 2009.
- ^ Fisher, B.S., N. Nakicenovic, K. Alfsen, J. Corfee Morlot, F. de la Chesnaye, J.-Ch. Hourcade, K. Jiang, M. Kainuma, E. La Rovere, A. Matysek, A. Rana, K. Riahi, R. Richels, S. Rose, D. van Vuuren, R. Warren, 2007: Chapter 3: Issues related to mitigation in the long term context, In Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Inter-governmental Panel on Climate Change [B. Metz, O.R. Davidson, P.R. Bosch, R. Dave, L.A. Meyer (eds)], Cambridge University Press, Cambridge,
- ^ Jackson, Robert B.; Abernethy, Sam; Canadell, Josep G.; Cargnello, Matteo; Davis, Steven J.; Féron, Sarah; Fuss, Sabine; Heyer, Alexander J.; Hong, Chaopeng; Jones, Chris D.; Damon Matthews, H.; O'Connor, Fiona M.; Pisciotta, Maxwell; Rhoda, Hannah M.; de Richter, Renaud (15 November 2021). "Atmospheric methane removal: a research agenda". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 379 (2210): 20200454. Bibcode:2021RSPTA.37900454J. doi:10.1098/rsta.2020.0454. ISSN 1364-503X. PMC 8473948. PMID 34565221.
- ^ "Coal Consumption Affecting Climate". Rodney and Otamatea Times, Waitemata and Kaipara Gazette. Warkworth, New Zealand. 14 August 1912. p. 7. Text was earlier published in Popular Mechanics, March 1912, p. 341.
- ^ Arrhenius, Svante (1896). "On the influence of carbonic acid in the air upon the temperature of the ground" (PDF). The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 41 (251): 237–276. doi:10.1080/14786449608620846. Archived (PDF) from the original on 18 November 2020. Retrieved 1 December 2020.
- ^ Arrhenius, Svante (1897). "On the Influence of Carbonic Acid in the Air Upon the Temperature of the Ground". Publications of the Astronomical Society of the Pacific. 9 (54): 14. Bibcode:1897PASP....9...14A. doi:10.1086/121158.
- ^ Easterbrook, Steve (18 August 2015). "Who first coined the term "Greenhouse Effect"?". Serendipity. Archived from the original on 13 November 2015. Retrieved 11 November 2015.
- ^ Ekholm N (1901). "On The Variations Of The Climate Of The Geological And Historical Past And Their Causes". Quarterly Journal of the Royal Meteorological Society. 27 (117): 1–62. Bibcode:1901QJRMS..27....1E. doi:10.1002/qj.49702711702.
- ^ Cook, J.; Nuccitelli, D.; Green, S.A.; Richardson, M.; Winkler, B.R.; Painting, R.; Way, R.; Jacobs, P.; Skuce, A. (2013). "Quantifying the consensus on anthropogenic global warming in the scientific literature". Environmental Research Letters. 8 (2): 024024. Bibcode:2013ERL.....8b4024C. doi:10.1088/1748-9326/8/2/024024.
- ^ Eddie Schwieterman. "Comparing the Greenhouse Effect on Earth, Mars, Venus, and Titan: Present Day and through Time" (PDF). Archived from the original (PDF) on 30 January 2015.
- ^ Scoping of the IPCC 5th Assessment Report Cross Cutting Issues (PDF). Thirty-first Session of the IPCC Bali, 26–29 October 2009 (Report). Archived (PDF) from the original on 9 November 2009. Retrieved 24 March 2019.
- ^ Hansen, James; Sato, Makiko; Russell, Gary; Kharecha, Pushker (2013). "Climate sensitivity, sea level and atmospheric carbon dioxide". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 371 (2001). 20120294. arXiv:1211.4846. Bibcode:2013RSPTA.37120294H. doi:10.1098/rsta.2012.0294. PMC 3785813. PMID 24043864.
External links
[edit] Media related to Greenhouse gases at Wikimedia Commons
Media related to Greenhouse gases at Wikimedia Commons- Carbon Dioxide Information Analysis Center (CDIAC), U.S. Department of Energy, retrieved 26 July 2020
- Annual Greenhouse Gas Index (AGGI) from NOAA
- Atmospheric spectra of GHGs and other trace gases. Archived 25 March 2013 at the Wayback Machine.
| |||||||||||||
| |||||||||||||
| |||||||||||||
| |||||||||||||
| |||||||||||||
| |||||||||||||





