 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌzaɪloʊˌmɛtəˈzoʊliːn/ ZY-lo-MET-ə-ZOH-leen |
| Trade names | Otrivin, Otrivine, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608026 |
| License data | |
| Pregnancy category |
|
| Dependence liability | Moderate[1] |
| Routes of administration | intranasal (spray or drops) |
| Drug class | α1 and α2 Adrenergic receptor agonist, Decongestant |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | >10 seconds |
| Excretion | Urinary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.629 |
| Chemical and physical data | |
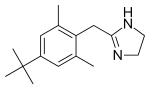
| Formula | C16H24N2 |
| Molar mass | 244.382 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Xylometazoline, also spelled xylomethazoline, is a medication used to reduce symptoms of nasal congestion, allergic rhinitis, and sinusitis.[2] Use is not recommended for more than seven days.[3] Use is also not recommended in those less than three months of age and some say not less than 6 years of age.[3][4] It is used directly in the nose as a spray or drops.[3]
Side effects include trouble sleeping, irritation of the nose, nausea, nosebleed (3%), period pain (10%) and headache (3%).[5][2][3] Long term use (> 10 days) is not recommended due to a rhinitis medicamentosa when stopped.[5][6] Use is not recommended during pregnancy.[2] Xylometazoline is in the decongestant and alpha-adrenergic agonist families of medication.[6][7]
One study classified it with selectivity ratios in alpha 2 adrenergic receptors of 151 for a2A vs a2B, 4.5 a2A vs a2C, and 33.9 a2B vs a2C. Making it a highly selective a2A agonist.[8]
Xylometazoline was patented in 1956 and came into medical use in 1959.[9][10] It is on the World Health Organization's List of Essential Medicines.[4][11] Xylometazoline is available as a generic medication.[3]
Mechanism of action
[edit]The drug works by stimulating adrenergic receptors on the lamina propria of blood vessels in the nose. The decongestant effect is due to constriction of large veins in the nose which swell up during the inflammation of any infection or allergy of the nose. The smaller arteries are also constricted and this causes the colour of the nasal epithelium to be visibly paler after dosage.
Xylometazoline is an imidazole derivative which is designed to mimic the molecular shape of adrenaline. It binds to α1 and α2 adrenergic receptors[12] in the nasal mucosa. Due to its sympathomimetic effects, it should not be used by people with high blood pressure, or other heart problems.
Extended usage of xylometazoline can result in decreased effectiveness or a buildup of tolerance against the drug.[13] The number of receptors decreases, and when the administration of the drug is ceased, chronic congestion can occur; this is called rhinitis medicamentosa, commonly referred to as rebound congestion. Moreover, long-term overdosing can cause degenerative changes in nasal mucous membranes that pose another health problem.[citation needed]
Society and culture
[edit]Brand names
[edit]The most common name for over-the-counter products containing xylometazoline internationally is "Otrivin" (used in Australia,[14] Canada, Estonia, Finland, Greece, Hungary, India,[15] Israel, Jordan, Netherlands, New Zealand, Norway, Poland, South Africa, Sweden, Vietnam, Hong Kong), "Otrivine" (United Kingdom, Ireland, Turkey, Belgium), or "Otriven" (Germany). A product marketed as "Otrivin Oxy" contains oxymetazoline instead of xylometazoline.
Other product names used include Antazol (Square, in Bangladesh), Xylomet (Opsonin, Bangladesh), Cirovin, Klarigen (in Denmark), Nasolin, Neo-Rinoleina, Novorin, Olynth, Otrinoz, Galazolin[16] (Russia, Ukraine, Belarus), Nasomist-X, Otrix, Rhinoset, Zenfresh, Naphthyzinium, Xymelyn (in Latvia), Sinutab Nasal Spray, Snup akut, Sudafed, Xylo-COMOD, Xylolin (in the United Arab Emirates), Xylovit, Olynth (in Serbia, the Czech Republic and Slovakia), Meralys (in Croatia) Xynosine (in Pakistan, Afghanistan, Kyrgyzstan and Kazakhstan), Xymelin, Zymelin, Xylostar, Xylorin (in Poland), Nasobol, Xylo Mepha and others (Switzerland), Decozal (in Jordan), Nasic, Orinox (Romania), Narhimed (Italy), nasa Rhinathiol (Belgium) and Zolinol,[17] Nasorhinathiol[18] and Vibrocil[19] (Portugal].
As of 2021, a number of consumer products containing xylometazoline are marketed in the United States.[20]
Formulations
[edit]The standard adult solution strength is 0.1% w/v xylometazoline (or 1 mg per 1 mL solution), and the dose for children under 12 is usually 0.05% (0.5 mg/mL).[21]
See also
[edit]References
[edit]- ^ ((Fulga, Ana, Andrei Zenovia, Doriana Cristea Ene, Constantin Stan, Dorel Firescu, and Iuliu Fulga. 2021. “Addiction to Nasal Decongestants Based on Α-Adrenoceptor Agonists Case Series and Literature Review: Array”. EuroEconomica 40 (2). https://dj.univ-danubius.ro/index.php/EE/article/view/1455))
- ^ a b c "Otrivine Adult Measured Dose Sinusitis Spray - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. 13 April 2016. Archived from the original on 29 December 2016. Retrieved 28 December 2016.
- ^ a b c d e British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 786. ISBN 978-0-85711-156-2.
- ^ a b World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ a b Eccles R, Martensson K, Chen SC (April 2010). "Effects of intranasal xylometazoline, alone or in combination with ipratropium, in patients with common cold". Current Medical Research and Opinion. 26 (4): 889–899. doi:10.1185/03007991003648015. PMID 20151787. S2CID 34728458.
- ^ a b Graf P (1997). "Rhinitis medicamentosa: aspects of pathophysiology and treatment". Allergy. 52 (40 Suppl): 28–34. doi:10.1111/j.1398-9995.1997.tb04881.x. PMID 9353558. S2CID 72326981.
- ^ "Xylometazoline nasal medical facts from Drugs.com". www.drugs.com. Archived from the original on 29 December 2016. Retrieved 28 December 2016.
- ^ Proudman RG, Akinaga J, Baker JG (October 2022). "The signaling and selectivity of α-adrenoceptor agonists for the human α2A, α2B and α2C-adrenoceptors and comparison with human α1 and β-adrenoceptors". Pharmacology Research & Perspectives. 10 (5): e01003. doi:10.1002/prp2.1003. PMC 9471048. PMID 36101495.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 552. ISBN 978-3-527-60749-5. Archived from the original on 29 December 2016.
- ^ US patent 2868802A, Hüni, Albrecht, "2-(γ-TERT-BUTYL-O,O'-DIMETHYL-PHENYL-METHYL)-IMIDAZOLINE AND SALTS", issued 1959-01-13, assigned to Ciba Pharmaceutical Products Inc., Summit, N. J.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Haenisch B, Walstab J, Herberhold S, Bootz F, Tschaikin M, Ramseger R, et al. (December 2010). "Alpha-adrenoceptor agonistic activity of oxymetazoline and xylometazoline". Fundamental & Clinical Pharmacology. 24 (6): 729–739. doi:10.1111/j.1472-8206.2009.00805.x. PMID 20030735. S2CID 25064699.
- ^ Gold Standard Clinical Pharmacology Archived 2008-05-25 at the Wayback Machine
- ^ "xylometazoline". Healthdirect Australia. Retrieved 25 October 2019.
- ^ "Otrivin | GSK Consumer Healthcare INDIA". Retrieved 17 June 2018.
- ^ "Галазолин (Galazolin) - инструкция по применению, состав, аналоги препарата, дозировки, побочные действия". rlsnet.ru. Retrieved 5 August 2019.
- ^ "Zolinol® - Edol". edol.pt. Retrieved 16 January 2024.
- ^ "Nasorhinathiol, 0,5 mg/mL-15 mL x 1 sol pulv nasal". farmaciasportuguesas.pt. Retrieved 16 January 2024.
- ^ "Vibrocil Actilong Mentol". vibrocil.pt. Retrieved 16 January 2024.
- ^ "FDA Drug Safety Communication: Serious adverse events from accidental ingestion by children of over-the-counter eye drops and nasal sprays". FDA. July 2021.
- ^ "Xylometazoline nasal Uses, Side Effects & Warnings". Archived from the original on 22 February 2015. Retrieved 22 February 2015.
External links
[edit]- "Xylometazoline". Drug Information Portal. U.S. National Library of Medicine.
Decongestants and other nasal preparations (R01) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Topical |
| ||||||||||
| Systemic use: Sympathomimetics | |||||||||||
| |||||||||||