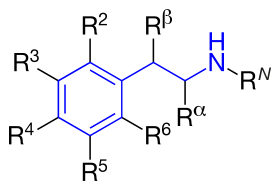
| Chemical
Structure
|
Short Name
|
RN
|
Rα
|
Rβ
|
R2
|
R3
|
R4
|
R5
|
Full Name
|
Biologic activity
|

|
meta-Tyramine |
|
|
|
|
OH |
|
|
3-hydroxyphenethylamine |
Trace amine
|
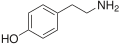
|
para-Tyramine |
|
|
|
|
|
OH |
|
4-hydroxyphenethylamine |
Trace amine
|
|
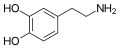
Dopamine |
|
|
|
|
OH |
OH |
|
3,4-dihydroxyphenethylamine |
Catecholamine neurotransmitter
|
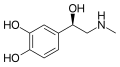
|
Epinephrine (Adrenaline) |
CH3 |
|
OH |
|
OH |
OH |
|
β,3,4-trihydroxy-N-methylphenethylamine |
Catecholamine neurotransmitter/Fight or Flight hormone
|

|
Norepinephrine (Noradrenaline) |
|
|
OH |
|
OH |
OH |
|
β,3,4-trihydroxyphenethylamine |
Catecholamine neurotransmitter/Fight or Flight hormone
|

|
Norfenefrine |
|
|
OH |
|
OH |
|
|
β,3-dihydroxyphenethylamine |
Trace amine
|
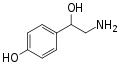
|
para-Octopamine |
|
|
OH |
|
|
OH |
|
β,4-dihydroxyphenethylamine |
Trace aminergic α-adrenoceptor agonist
|

|
Oxidopamine |
|
|
|
OH |
|
OH |
OH |
2,4,5-trihydroxyphenethylamine |
neurotoxic agent for the dopamine and norepinephrine receptors
|

|
Phenylephrine |
CH3 |
|
OH |
|
OH |
|
|
β,3-dihydroxy-N-methylphenethylamine |
α-adrenergic agonist; decongestant
|
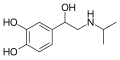
|
Isoprenaline
|
CH(CH3)2
|
|
OH
|
|
OH
|
OH
|
|
β,3-dihydroxy-N-isopropylphenethylamine
|
β-adrenergic agonist; decongestant
|
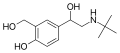
|
Salbutamol |
C(CH3)3 |
|
OH |
|
CH2OH |
OH |
|
β,4-dihydroxy-3-hydroxymethyl-N-tert-butylphenethylamine |
Short-action β2-adrenergic agonist
|

|
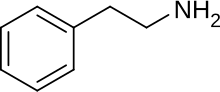
β-Methylphenethylamine |
|
|
CH3 |
|
|
|
|
β-methylphenethylamine |
Stimulant
|

|
Amphetamine |
|
CH3 |
|
|
|
|
|
α-methylphenethylamine |
Monoamine releasing agent; Stimulant
|
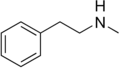
|
N-Methylphenethylamine |
CH3 |
|
|
|
|
|
|
N-methylphenethylamine |
Trace amine; endogenous amphetamine isomer
|

|
N,N-Dimethylphenethylamine |
(CH3)2 |
|
|
|
|
|
|
N,N-dimethylphenethylamine |
Trivial effects (used as a food additive and flavoring agent)
|
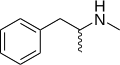
|
Methamphetamine |
CH3 |
CH3 |
|
|
|
|
|
N-methylamphetamine; N,α-dimethylphenethylamine |
Monoamine releasing agent; stimulant; neurotoxin
|
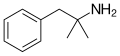
|
Phentermine |
|
(CH3)2 |
|
|
|
|
|
α-methylamphetamine; α,α-dimethylphenethylamine |
Stimulant, anorectic
|
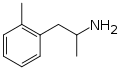
|
Ortetamine |
|
CH3 |
|
CH3 |
|
|
|
2-methylamphetamine; 2,α-dimethylphenethylamine |
Stimulant, anorectic
|
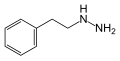
|
Phenelzine
|
NH2
|
|
|
|
|
|
|
β-phenylethylhydrazine
|
Monoamine oxidase inhibitor
|
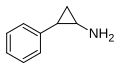
|
Tranylcypromine
|
|
-CH2-
|
|
|
|
|
2-phenylcyclopropylamine
|
Monoamine oxidase inhibitor
|

|
Selegiline
|
-CH2-C≡CH
|
CH3
|
|
|
|
|
|
N,α-dimethyl-N-2-propynylphenethylamine
|
MAO-B selective monoamine oxidase inhibitor
|

|
Methylphenidate |
-CH2-CH2-CH2-CH2- |
C(OCH3)=O |
|
|
|
|
N,α-butylene-β-methoxycarbonylphenethylamine |
NDRI; Stimulant
|

|
Ephedrine / Pseudoephedrine |
CH3 |
CH3 |
OH |
|
|
|
|
N-methyl-β-hydroxyamphetamine |
Releasing agent; stimulant; decongestant
|

|
Cathine |
|
CH3 |
OH |
|
|
|
|
d-β-hydroxyamphetamine |
Moderately selective norepinephrine releasing agent
|
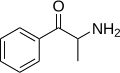
|
Cathinone |
|
CH3 |
=O |
|
|
|
|
β-ketoamphetamine |
Selective norepinephrine and dopamine releasing agent
|
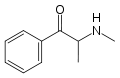
|
Methcathinone |
CH3 |
CH3 |
=O |
|
|
|
|
N-methylcathinone |
Selective norepinephrine and dopamine releasing agent
|
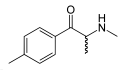
|
Mephedrone |
CH3 |
CH3 |
=O |
|
|
CH3 |
|
4-methylmethcathinone |
Stimulant, unknown pharmacodynamic actions
|
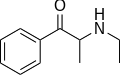
|
Ethcathinone |
CH2CH3 |
CH3 |
=O |
|
|
|
|
N-ethylcathinone |
Stimulant and norepinephrine releasing agent
|
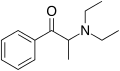
|
Amfepramone (diethylpropion) |
C2H5, C2H5[note 2] |
CH3 |
=O |
|
|
|
|
N-diethyl-β-ketoamphetamine |
Anorectic
|
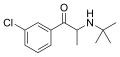
|
Bupropion |
C(CH3)3 |
CH3 |
=O |
|
|
|
Cl |
5-chloro-N-tert-butyl-β-ketoamphetamine |
NDRI
|
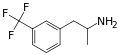
|
Norfenfluramine |
|
CH3 |
|
|
CF3 |
|
|
3-trifluoromethyl-amphetamine |
SSRA
|

|
Fenfluramine |
CH2CH3 |
CH3 |
|
|
CF3 |
|
|
3-trifluoromethyl-N-ethylamphetamine |
SSRA
|
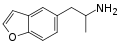
|
5-APB |
|
CH3 |
|
|
-CH=CH-O- |
|
5-(2-aminopropyl)benzofuran |
Stimulant, entactogen
|

|
6-APB |
|
CH3 |
|
|
-O-CH=CH- |
|
6-(2-aminopropyl)benzofuran |
Stimulant, entactogen
|

|
MDA |
|
CH3 |
|
|
-O-CH2-O- |
|
3,4-methylenedioxy-amphetamine |
Stimulant, psychedelic, entactogen
|
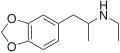
|
MDEA |
CH2CH3 |
CH3 |
|
|
-O-CH2-O- |
|
3,4-methylenedioxy-N-ethylamphetamine |
Psychedelic, entactogen, and releasing agent
|

|
MDMA |
CH3 |
CH3 |
|
|
-O-CH2-O- |
|
3,4-methylenedioxy-N-methylamphetamine |
Psychedelic, entactogen, and releasing agent
|

|
MDMC |
CH3 |
CH3 |
=O |
|
-O-CH2-O- |
|
3,4-methylenedioxymethcathinone |
Psychedelic, entactogen, and releasing agent
|

|
MMDA |
|
CH3 |
|
|
-O-CH2-O- |
OCH3 |
5-methoxy-3,4-methylenedioxy-amphetamine |
Stimulant, psychedelic and entactogen
|

|
MMDMA |
CH3 |
CH3 |
|
|
-O-CH2-O- |
OCH3 |
5-methoxy-3,4-methylenedioxy-N-methylamphetamine |
Psychedelic, entactogen, and releasing agent
|

|
Lophophine |
|
|
|
|
-O-CH2-O- |
OCH3 |
5-methoxy-3,4-methylenedioxyphenethylamine |
Psychedelic and entactogen
|
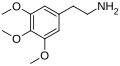
|
Mescaline |
|
|
|
|
OCH3 |
OCH3 |
OCH3 |
3,4,5-trimethoxyphenethylamine |
Psychedelic and entactogen
|

|
Proscaline |
|
|
|
|
OCH3 |
OCH2CH2CH3 |
OCH3 |
2-(3,5-dimethoxy-4-propoxyphenyl)ethanamine |
Psychedelic and entactogen
|

|
Metaescaline |
|
|
|
|
OCH2CH3 |
OCH3 |
OCH3 |
2-(3-ethoxy-4,5-dimethoxyphenyl)ethanamine |
Psychedelic and entactogen
|
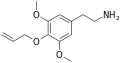
|
Allylescaline |
|
|
|
|
OCH3 |
OCH2CH1CH2 |
OCH3 |
4-Allyloxy-3,5-dimethyloxyphenylethylamine |
Psychedelic and entactogen
|

|
Methallylescaline |
|
|
|
|
OCH3 |
OCH2C(CH2CH3) |
OCH3 |
4-Methallyloxy-3,5-dimethoxyphenethylamine |
Psychedelic and entactogen
|

|
Asymbescaline |
|
|
|
|
OCH2CH3 |
OCH2CH3 |
OCH3 |
3,4-Diethoxy-5-methoxyphenethylamine |
Psychedelic and euphoriant
|

|
DOM |
|
CH3 |
|
OCH3 |
|
CH3 |
OCH3 |
2,5-dimethoxy-4-methylamphetamine |
Psychedelic
|
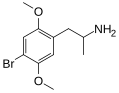
|
DOB |
|
CH3 |
|
OCH3 |
|
Br |
OCH3 |
2,5-dimethoxy-4-bromoamphetamine |
Psychedelic
|
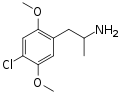
|
DOC |
|
CH3 |
|
OCH3 |
|
Cl |
OCH3 |
2,5-dimethoxy-4-chloroamphetamine |
Psychedelic
|
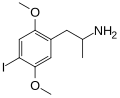
|
DOI |
|
CH3 |
|
OCH3 |
|
I |
OCH3 |
2,5-dimethoxy-4-iodoamphetamine |
Psychedelic
|
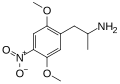
|
DON |
|
CH3 |
|
OCH3 |
|
NO2 |
OCH3 |
2,5-dimethoxy-4-nitroamphetamine |
Stimulant
|
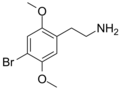
|
2C-B |
|
|
|
OCH3 |
|
Br |
OCH3 |
2,5-dimethoxy-4-bromophenethylamine |
Psychedelic, stimulant, entactogen and euphoriant
|
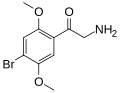
|
βk-2C-B |
|
|
=O |
OCH3 |
|
Br |
OCH3 |
2,5-dimethoxy-4-bromo-β-ketophenethylamine |
Psychedelic, stimulant, entactogen and euphoriant
|

|
2C-C |
|
|
|
OCH3 |
|
Cl |
OCH3 |
2,5-dimethoxy-4-chlorophenethylamine |
Psychedelic
|
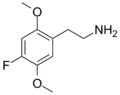
|
2C-F |
|
|
|
OCH3 |
|
F |
OCH3 |
2,5-dimethoxy-4-fluorophenethylamine |
Psychedelic
|

|
2C-I |
|
|
|
OCH3 |
|
I |
OCH3 |
2,5-dimethoxy-4-iodophenethylamine |
Psychedelic, stimulant
|

|
2C-D |
|
|
|
OCH3 |
|
CH3 |
OCH3 |
2,5-dimethoxy-4-methylphenethylamine |
Psychedelic, stimulant
|

|
2C-E |
|
|
|
OCH3 |
|
CH2-CH3 |
OCH3 |
2,5-dimethoxy-4-ethylphenethylamine |
Psychedelic
|
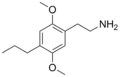
|
2C-P |
|
|
|
OCH3 |
|
CH2-CH3-CH3 |
OCH3 |
2,5-dimethoxy-4-propylphenethylamine |
Entactogen, euphoriant and Psychedelic
|
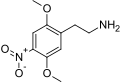
|
2C-N |
|
|
|
OCH3 |
|
NO2 |
OCH3 |
2,5-dimethoxy-4-nitrophenethylamine |
euphoriant
|
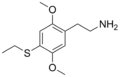
|
2C-T-2 |
|
|
|
OCH3 |
|
S-CH2CH3 |
OCH3 |
2,5-dimethoxy-4-ethylthio-phenethylamine |
Psychedelic
|
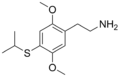
|
2C-T-4 |
|
|
|
OCH3 |
|
S-CH(CH3)2 |
OCH3 |
2,5-dimethoxy-4-isopropylthio-phenethylamine |
Psychedelic
|

|
2C-T-7 |
|
|
|
OCH3 |
|
S-CH2CH2CH3 |
OCH3 |
2,5-dimethoxy-4-propylthio-phenethylamine |
Psychedelic
|
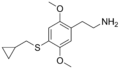
|
2C-T-8 |
|
|
|
OCH3 |
|
S-CH2-C3H5 |
OCH3 |
2,5-dimethoxy-4-cyclopropylmethylthio-phenethylamine |
Psychedelic
|

|
2C-T-19 |
|
|
|
OCH3 |
|
S-C(CH3)3 |
OCH3 |
2,5-dimethoxy-4-tert-butylthio-phenethylamine |
Psychedelic
|

|
2C-T-21 |
|
|
|
OCH3 |
|
S-CH2-CH2-F |
OCH3 |
2,5-dimethoxy-4-(2-fluoroethylthio)-phenethylamine |
Psychedelic and euphoriant
|

|
25B-NBOMe[3] |
CH2-C6H4-OCH3 |
|
|
OCH3 |
|
Br |
OCH3 |
2-(4-bromo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine |
Psychedelic
|
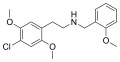
|
25C-NBOMe |
CH2-C6H4-OCH3 |
|
|
OCH3 |
|
Cl |
OCH3 |
2-(4-chloro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine |
Psychedelic
|
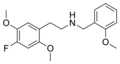
|
25F-NBOMe |
CH2-C6H4-OCH3 |
|
|
OCH3 |
|
F |
OCH3 |
2-(4-fluoro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine |
Psychedelic
|
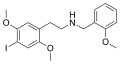
|
25I-NBOMe |
CH2-C6H4-OCH3 |
|
|
OCH3 |
|
I |
OCH3 |
2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine |
Psychedelic
|

|
25D-NBOMe |
CH2-C6H4-OCH3 |
|
|
OCH3 |
|
CH2 |
OCH3 |
2-(4-methyl-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine |
Psychedelic
|

|
25E-NBOMe |
CH2-C6H4-OCH3 |
|
|
OCH3 |
|
CH2-CH3 |
OCH3 |
2-(4-ethyl-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine |
Psychedelic
|

|
25P-NBOMe |
CH2-C6H4-OCH3 |
|
|
OCH3 |
|
CH2-CH3-CH3 |
OCH3 |
2-(4-propyl-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine |
Psychedelic
|
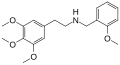
|
Mescaline-NBOMe |
CH2-C6H4-OCH3 |
|
|
|
OCH3 |
OCH3 |
OCH3 |
N-(2-Methoxybenzyl)-2-(3,4,5-trimethoxyphenyl)ethanamine |
Psychedelic
|
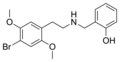
|
25B-NBOH |
CH2–C6H4–OH |
|
|
OCH3 |
|
Br |
OCH3 |
N-(2-hydroxybenzyl)-2,5-dimethoxy-4-bromo-phenethylamine |
Psychedelic
|

|
25C-NBOH |
CH2–C6H4–OH |
|
|
OCH3 |
|
Cl |
OCH3 |
N-(2-hydroxybenzyl)-2,5-dimethoxy-4-chloro-phenethylamine |
Psychedelic
|
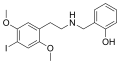
|
25I-NBOH |
CH2–C6H4–OH |
|
|
OCH3 |
|
I |
OCH3 |
N-(2-hydroxybenzyl)-2,5-dimethoxy-4-iodo-phenethylamine |
Psychedelic
|
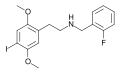
|
25I-NBF |
CH2–C6H4–F |
|
|
OCH3 |
|
I |
OCH3 |
N-(2-fluorobenzyl)-2,5-dimethoxy-4-iodo-phenethylamine |
Psychedelic
|
|
|
Short Name
|
RN
|
Rα
|
Rβ
|
R2
|
R3
|
R4
|
R5
|
Full Name
|
Biologic activity
|