Substituted phenylmorpholines, or substituted phenmetrazines alternatively, are chemical derivatives of phenylmorpholine or of the psychostimulant drug phenmetrazine. Most such compounds act as releasers of monoamine neurotransmitters, and have stimulant effects. Some also act as agonists at serotonin receptors, and compounds with an N-propyl substitution act as dopamine receptor agonists. A number of derivatives from this class have been investigated for medical applications, such as for use as anorectics or medications for the treatment of ADHD. Some compounds have also become subject to illicit use as designer drugs.[1][2][3][4]



| Substance | Structure | CAS number |
|---|---|---|
| 2-phenylmorpholine |  |
23972-41-0 |
| 2-phenyl-3-methylmorpholine (phenmetrazine) |  |
134-49-6 |
| 2-phenyl-3,4-dimethylmorpholine (phendimetrazine) |  |
634-03-7 |
| 2-phenyl-5-methylmorpholine (isophenmetrazine) | 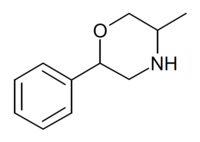 |
80123-66-6 |
| 2-phenyl-3-ethylmorpholine (phenetrazine) |  |
100368-98-7 |
| 2-phenyl-3-methyl-4-ethylmorpholine (phenmetetrazine) |  |
92196-09-3 |
| 2-phenyl-3,5-dimethylmorpholine (PDM-35) | 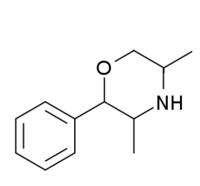 |
1218345-44-8 |
| 2-phenyl-3,6-dimethylmorpholine (6-methylphenmetrazine, 3,6-DMPM) |  |
92902-99-3 |
| 2-phenyl-5,5-dimethylmorpholine (G-130) |  |
42013-48-9 |
| 2-phenyl-3-methylmorpholin-5-one (fenmetramide) |  |
5588-29-4 |
| 4-isopropyl-2-phenylmorpholine[5] | 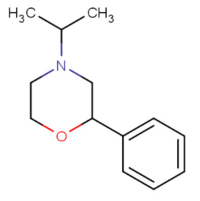 |
23222-62-0 |
| Fenbutrazate |  |
4378-36-3 |
| Morazone |  |
6536-18-1 |
| 2-Fluorophenmetrazine |  |
1533654-24-8 |
| 3-fluorophenmetrazine (PAL-593) | 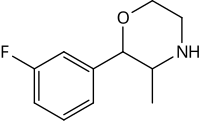 |
1350768-28-3 |
| 4-Fluorophenmetrazine (PAL-748) |  |
1097796-73-0 |
| 3-fluorophenetrazine |  |
|
| 3-chlorophenmetrazine (PAL-594) |  |
1097796-78-5 |
| 3-Methoxyphenmetrazine |  |
|
| 2-Methylphenmetrazine |  |
1507705-48-7 |
| 3-methylphenmetrazine (PAL-773) |  |
1350768-41-0 |
| 4-methylphenmetrazine (PAL-747) |  |
1998216-41-3 |
| 4-methylphendimetrazine |  |
1445576-23-7 |
| 3,4-Methylenedioxyphenmetrazine[6] |  |
|
| Naphthylmetrazine (PAL-704) |  |
|
| 2-(thiophen-2-yl)-3-methylmorpholine | 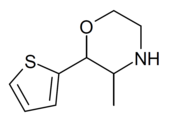 |
|
| 2-(2,5-dimethoxy-4-bromophenyl)morpholine[7] |  |
|
| 2-(3-(Trifluoromethyl)phenyl)morpholine (flumexadol)[8] | 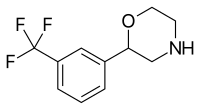 |
30914-89-7 |
| Oxaflozane |  |
26629-87-8 |
| Phenmetrazol |  |
1350768-19-2 |
| N-Ethylphenmetrazol |  |
97630-98-3 |
| (+)-(2S,3S)-2-(3-chlorophenyl)-3,5,5-trimethylmorpholin-2-ol (radafaxine) |  |
106083-71-0 |
| (2S,3S,5R)-2-(3,5-difluorophenyl)-3,5-dimethylmorpholin-2-ol (manifaxine) |  |
135306-39-7 |
| PF-219,061[9] | 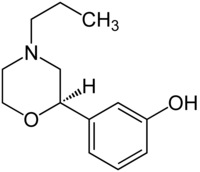 |
710654-74-3 |
| PF-592,379 |  |
710655-15-5 |
| OSU-6162 | 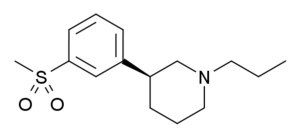 |
156907-84-5 |
| LPH-5[10] |  |
2641630-97-7 |
| 2-Methyl-3-phenylpiperidine |  |
70769-67-4 |
| 2-Phenyl-3-methyl-thiomorpholine |  |
|
| Picilorex | 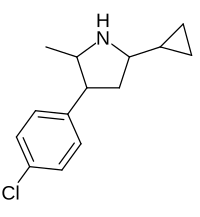 |
62510-56-9 |
| Butyltolylquinuclidine | 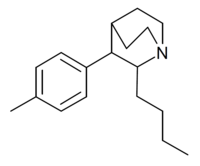 |
|
| 3-benzylmorpholine (3-BZM)[11] | 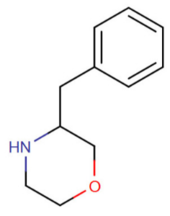 |
7684-27-7 |
| 3-Benzhydrylmorpholine (3-BZHM) |  |
93406-27-0 |
See also
[edit]- Substituted amphetamine
- Substituted benzofuran
- Substituted cathinone
- Substituted methylenedioxyphenethylamine
- List of aminorex analogues
- List of methylphenidate analogues
- List of phenyltropanes
- List of cocaine analogues
References
[edit]- ^ Boswell GE (1997). "Synthesis, stereochemistry and anti-tetrabenazine activity of bicyclo analogues of 2-phenylmorpholines". Journal of Heterocyclic Chemistry. 34 (6): 1813–1820. doi:10.1002/jhet.5570340629.
- ^ US 20130203752, Blough BE, Rothman R, Landavazo A, Page KM, Decker AM, "Phenylmorpholines and analogues thereof", published 8 August 2013
- ^ Mayer FP, Burchardt NV, Decker AM, Partilla JS, Li Y, McLaughlin G, et al. (May 2018). "Fluorinated phenmetrazine "legal highs" act as substrates for high-affinity monoamine transporters of the SLC6 family". Neuropharmacology. 134 (Pt A): 149–157. doi:10.1016/j.neuropharm.2017.10.006. PMC 7294773. PMID 28988906.
- ^ McLaughlin G, Baumann MH, Kavanagh PV, Morris N, Power JD, Dowling G, et al. (September 2018). "Synthesis, analytical characterization, and monoamine transporter activity of the new psychoactive substance 4-methylphenmetrazine (4-MPM), with differentiation from its ortho- and meta- positional isomers". Drug Testing and Analysis. 10 (9): 1404–1416. doi:10.1002/dta.2396. PMC 7316143. PMID 29673128.
- ^ Chem-Sink (chemicals that are Sn2 products & Friedel-Crafts alkylation reactants)
- ^ Swist M, Wilamowski J, Zuba D, Kochana J, Parczewski A (May 2005). "Determination of synthesis route of 1-(3,4-methylenedioxyphenyl)-2-propanone (MDP-2-P) based on impurity profiles of MDMA". Forensic Science International. 149 (2–3): 181–92. doi:10.1016/j.forsciint.2004.06.016. PMID 15749360.
- ^ Glennon RA, Bondarev ML, Khorana N, Young R, May JA, Hellberg MR, et al. (November 2004). "Beta-oxygenated analogues of the 5-HT2A serotonin receptor agonist 1-(4-bromo-2,5-dimethoxyphenyl)-2-aminopropane". Journal of Medicinal Chemistry. 47 (24): 6034–41. doi:10.1021/jm040082s. PMID 15537358.
- ^ Chemicalbook dot com: Flumexadol
- ^ Morpholine Dopamine Agonists For The Treatment Of Pain. Michael Andrew Ackley US 2009/0318451 AI Dec., 24th 2009. 1st Page.
- ^ Kristensen J, et al. 5-HT2A Agonists for Use in Treatment of Depression. Patent US 2021/0137908
- ^ NIH PubChem Compound Summary for CID 3283983
External links
[edit] Media related to Substituted phenylmorpholines at Wikimedia Commons
Media related to Substituted phenylmorpholines at Wikimedia Commons
| α1 |
| ||||
|---|---|---|---|---|---|
| α2 |
| ||||
| β |
| ||||
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|