This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages)
This article relies excessively on references to primary sources. Please improve this article by adding secondary or tertiary sources. Find sources: "Dichloropane" – news · newspapers · books · scholar · JSTOR (February 2016) (Learn how and when to remove this message)
This article may be too technical for most readers to understand. Please help improve it to make it understandable to non-experts, without removing the technical details. (February 2016) (Learn how and when to remove this message)
(Learn how and when to remove this message)
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
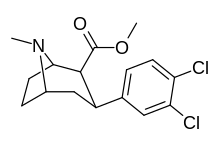
| Formula | C15H17Cl2NO2 |
| Molar mass | 314.21 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dichloropane ((−)-2β-Carbomethoxy-3β-(3,4-dichlorophenyl)tropane, RTI-111, O-401) is a stimulant of the phenyltropane class that acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) with IC50 values of 3.13, 18, and 0.79 nM, respectively.[1] In animal studies, dichloropane had a slower onset and longer duration of action compared to cocaine.[2][3]
Methylecgonidine is the direct precursor to this compound.[4]